Physical Properties of Soil
Soil possesses many characteristic physical properties such as density, porosity, permeability, temperature, water and atmosphere, each of which can be studied under the following separate headings:
- Soil density: The average density of soil is 2.65 gms. per ml. The density of soil varies greatly depending upon the degree of weathering.
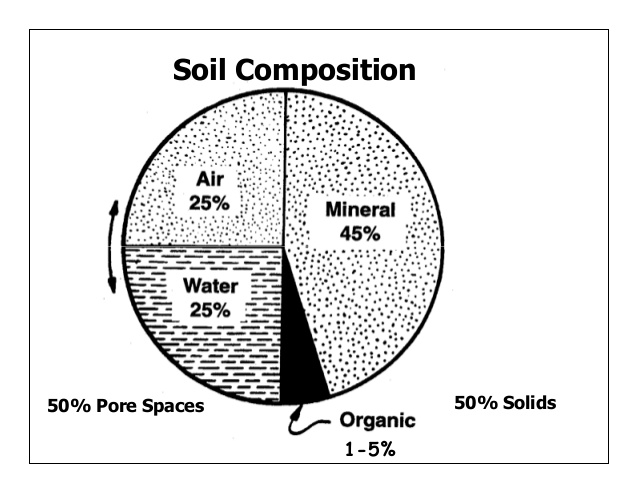
- Porosity: The spaces present between soil particles in a given volume of soil is called pore spaces. The percentage of soil volume occupied by pore space or by the interstitial space is called the porosity of the soil. The porosity of soil depends upon the texture and structure compactness and organic contents of the soil. The porosity of the soil increases with the increase in the percentage of organic matter in the soil. The pore spaces are of two types–
(a) Micro-pore spaces (capillary pore spaces) and (b) Macropore spaces (non-capillary pore spaces). Capillary pore spaces can hold more water and restrict the free movement of water and air to a considerable extent, whereas macro-pore spaces have little water holding capacity and allow free movement of moisture and air in the soil under normal conditions.
- Permeability of soil: The characteristic of soil that determines the movement of water through pore spaces is known as soil permeability. Soil permeability is directly dependent on the pore size, therefore, it is higher for loose soil with a large number of macro-pore spaces than it is for compact soil with numerous micro-pore spaces.
- Soil temperature: Soil gets heat energy from different sources such as solar radiation, decomposing organic matter, and heat formed in the interior of the earth. The temperature of the soil is affected by its colour, texture, water content, slope, altitude of the land and also by climate and vegetational cover of the soil. Evaporation of water from soil makes it cooler. Black soils absorb more heat than white soils. Sandy soils absorb more heat and radiate it out quickly at night than clay or loam soils. The soil temperature greatly affects the Physio-chemical and biological processes in the soil. For example, the germination of seeds, normal growth of roots and biological activity of soil-inhabiting micro-and macro-organisms require proper and specific temperature.
- Soil water: In soil, water is not only important as a solvent and transporting agent but in various ways, it maintains soil texture, arrangement and compactness of soil particles and makes soil habitat livable for plants and animals. It comes in soil mainly through the infiltration of precipitated water (dew, rain, sleet, snow, and hail) and irrigation. According to the improved classification method of Bouyoucos (1920), soil water is classified into the following types- gravitational and groundwater, capillary water, hygroscopic water, combined water and water vapour.
(i) Gravitational water: In a well-saturated soil, the accessory (extra) amount of water displaces air from the pore spaces between soil particles and percolates downwardly under the gravitational influence and finally, it is accumulated in the pore spaces. This accumulated excess water of large soil spaces is called gravitational water. When this gravitational water further percolates down and reaches the level of parent rock, it is called groundwater. Both kinds of these soil water are ecologically important in the leaching of nutrients.
(ii) Capillary water: The water which is held by capillary forces (i.e., surface tension and attraction forces of water molecules) in smaller soil channels, when the gravitational water and groundwater have been drained, is called capillary water. Capillary water occurs as a thin film around soil particles in the capillary spaces and represents the normally available water to the plants. It remains in the soil for long periods and carries with it nutrients in solution. Humus has more capillary water than soil minerals.
(iii) Hygroscopic water: Soil particles retain some water so firmly that the plants cannot absorb this. Such soil water is called hygroscopic water.
(iv) Combined water: The combined water is the water of chemical compounds held by chemical forces of molecules such as hydroxides of silicon, iron and aluminium. It is of no ecological significance.
(v) Water vapour: Some soil water occurs as moisture or water vapours in the soil atmosphere. Further, the total amount of water present in the soil is called holard. The quantity of water that plant roots can absorb out of holard is called chresard and that amount of soil water which cannot be absorbed by plant roots is called echard. Moreover, there are some terms that reflect the water status of soil and are generally used for comparative studies of different soils.
(a) Soil water potential: This is an expression of the total reduction of water potential in the soil, due to mineral matrix, solubility, eternal pressure and gravitation effects.
(b) Field capacity: When soil holds all the water it can, but no gravitational water, it is said to be at its field capacity. Field capacity is generally defined as the water content of undisturbed soil (% oven-dry weight) after it is saturated by rainfall and drainage of gravitational water has completely stopped. The field capacity of soil may be taken as the total amount of capillary, hygroscopic and combined water plus water vapour.
(c) Moisture equivalent: It is defined as the water content (% oven dry weight) retained by the undisturbed soil.
(d) Water holding capacity (or storage capacity): This is the extent to which soil can hold capillary water against gravity. It is equal to field capacity less hygroscopic water.
(e) Hygroscopic-coefficient: It is defined as the water vapour (%) absorbed by the unit weight of dry soil when placed in an atmosphere completely saturated with water vapours.
(f) Permanent wilting percentage (or wilting coefficient): It is the amount of water (% oven dry weight) that remains in soil when permanent wilting is present in the plants growing in the soil. The status of soil water is also represented in terms of capillary potential (matric potential). The relationship that exists between soil water content and water potential is usually determined by a pressure membrane apparatus, called a tensiometer.
- Soil atmosphere: Gases found in pore spaces of soil profiles form the soil atmosphere. The soil atmosphere contains three main gases namely O2, CO2, and N2. Soil air differs from atmospheric air in having more moisture and CO2 and less O2. The soil atmosphere is affected by temperature, atmospheric pressure, wind, rainfall, etc. Loam soils with humus contain a normal proportion of air and water (about 34% air and 66% water) and therefore are good for the majority of crops. Course-textured or well-structured soils contain higher gaseous diffusive transfer rates than fine-textured or poorly-structured soils under wet conditions. This is because they contain many large pores which remain gas-filled. The water-filled pores of the fine-textured soils form potent barriers to gaseous diffusion; O2 diffuses ten thousand times more slowly in water than in gas. In dry soil, the situation is reversed, as the fine-textured soils have a greater total pore space and provide a larger gas-filled cross-sectional area for diffusion. Soil aeration is very important in the growth of roots, seed germination and microbial activity. Poor soil aeration suppresses root hair development and may reduce the rate of absorption of water and nutrients.
- Soil solution: There exists a weak solution of various salts, along with other liquids and gases in the soil mass. This soil solution contains almost all the essential minerals. A complete mixture of minerals such as carbonates, sulphates, nitrates, chlorides, and organic salts of Ca, Mg, Na, K, etc., are found dissolved in water. The chemical nature of soil solution depends on the nature of parent matter, chemical nature of organic matter and climatic factors and other factors involved in pedogenesis. The soil solution is the primary source of inorganic nutrients for plant roots. Soils with an optimal concentration of various nutrient solutes are called eutrophic, whereas those with suboptimal concentrations of these nutrient salts are called oligotrophic.
Chemical Properties of Soil
Soil is a mixture of various inorganic and organic chemical compounds and exhibits certain significant chemical properties, all of which can be discussed as follows :
(a) Inorganic elements and compounds of soil: The chief inorganic constituents of soils are the compounds of the following elements—Ai, Si, Ca, Mg, Fe, K and Na. Soil also contains smaller amounts of compounds of the following inorganic elements—B, Mn, Cu, Zn, Mo, Co, I, F, etc.
(b) Organic matter of the soil: The chief organic component of soil is humus which chemically contains amino acids, proteins, purines, pyrimidines, aromatic compounds, hexose sugars, sugar alcohols, methyl sugars, fats, oils, waxes, resins, tannins, lignin and some pigments. Further, humus is a black-coloured, odourless, homogeneous complex substance.
(c) Colloidal properties: As soil is composed of crystalloids and colloids, therefore, it exhibits all the Physico-chemical properties which are related to these two soil particles. Colloids for example exhibit absorption, electrical properties, coagulation, Tyndal phenomenon, Brownian movement, dialysis, etc.
(d) Soil pH: Many chemical properties of soils centre around soil reaction. As regards their nature, some soils are neutral, some are acidic and some basic. The acidity, alkalinity and neutrality of soils are described in terms of hydrogen-ion concentrations or pH values. A pH value of 7.0 indicates neutrality, a value above this figure (7.1–14.00) indicates alkaline condition and a value below (0–6.9) indicates acid conditions. Normally, the pH value of soils lies between 2.2 and 9.6. In India, acidic soils (pH below 5.5 to 5.6) occur in the high rainfall areas of Western ghats, Kerala, Eastern Orissa, West Bengal, Tripura, Manipur and Assam. The saline, alkaline or basic soils (called ‘Usar’, contain pH up to 8.5) of India, occur in U.P., West Bengal, Punjab, Bihar, Orissa, Maharashtra, Madras, M.P., A.P., Gujarat, Delhi and Rajasthan.
Some pH values are ecologically significant for the plants (Pearsall, 1952); plants regarded as calcicoles usually occur in soil with a pH of 6.5, whereas calcifuges occur in soil with a pH value below 3.8–4.0. Soils above pH 6.5 are generally cation-saturated (those containing free CaCO3 are called calcareous soils) while soil below pH 3.8-4.0 contains a considerable content of exchangeable hydrogen. These limits of pH value are also reflected in the nature of the soil’s organic matter: raw humus or more which is associated with soils below pH 3.8, while mull is characteristic of the more cation saturated soils of pH 4.8–5.0 and above. Highly acidic and highly saline or alkaline soils often remain injurious for plant growth, microorganisms, etc. Soil pH strongly affects the microbial activities, as at below pH 5.0 bacterial as well as fungal activities are reduced. Neutral or slightly acidic soil, however, remains best for the growth of the majority of plants.