Synaptic transmission
Introduction
- By means of an electrical impulse, a neuron’s function is to communicate or relay information to another cell. The site at which the impulse is transferred from one cell to the next is a synapse. A muscle cell, glandular cell, or other neuron may terminate with a neuron.
- Transmission of neuron-to-neuron: The presynaptic neuron transmits the impulse towards the synapse at these types of synapses and the postsynaptic neuron transmits the impulse away from the synapse.
- In particular, it is the presynaptic neuron axon terminal that comes into contact with the body of the cell or the postsynaptic neuron dendrites. Thousands of inputs are received by most neurons, particularly in the CNS.
- The transmission of the impulse at the synapse is, as will become evident, only postsynaptic neuron activity is influenced by unidirectional and presynaptic neurons.
Chemical synapses
- The majority of nervous system synapses are chemical synapses in which the presynaptic neuron and the postsynaptic neuron are not in direct contact, but are separated by a narrow space called the synaptic cleft (0.01 to 0.02 m).
- This space prevents the electrical impulse from being spread directly from one cell to the next. A chemical known as a neurotransmitter is released from the presynaptic neuron instead. The neurotransmitter diffuses throughout the synaptic cleft, binds to its specific receptor, and alters the postsynaptic neuron’s electrical activity.
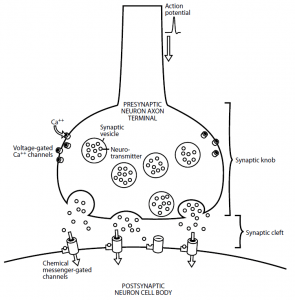
- In Figure 1, the mechanism of action of a chemical synapse is shown. To form a swelling referred to as the synaptic knob, the axon terminal broadens.
- Many synaptic vesicles that store the preformed neurotransmitter are within the synaptic knob. There are voltage-gated Ca++ channels also found in the membrane of the synaptic knob.
- The accompanying change in voltage causes the voltage-gated Ca++ channels to open when the electrical impulse, or action potential, has been transmitted along the axon length and reaches the axon terminal.
- Compared to the intracellular fluid, because calcium is higher in the extracellular fluid, Ca++ ions enter the cell down their concentration gradient. By causing the vesicles to fuse with the presynaptic membrane, the Ca++ ions then induce the release of the neurotransmitter from the synaptic vesicles into the synaptic cleft, thus facilitating the exocytosis process.
- Throughout the cleft, the neurotransmitter molecules diffuse and bind to specific receptors on the postsynaptic neuron membrane. This neurotransmitter binding transforms the postsynaptic neuron’s permeability to one or more ions.
- As always, a change in the permeability of the ion results in a change in the potential of the cell membrane. This synapse change occurs only in the form of a graded potential.
- The change in membrane potential at any given synapse is not great enough to reach the threshold and generate the potential for action. Instead, toward the axon hillock, many graded potential generated at one or more synapses are carried out over the cell membrane.
- If the depolarization caused by the addition of multiple graded potentials together is sufficient to reach the threshold of the axon hillock, an action potential is generated here.
The two types of synapses are:
- Excitatory synapses
- Inhibitory synapses
- Binding the neurotransmitter to its receptor at an excitatory synapse increases membrane permeability to Na+ ions and K+ ions via chemical messenger-gated channels closely associated with the receptor.
- As a result , the concentration and electrical gradients of Na+ ions enter the cell and K+ ions only leave the cell below its concentration gradient. Because two forces cause sodium inward diffusion and only one force causes potassium outward diffusion, the Na+ ion influx is significantly greater than the K+ ion efflux.
- This greater movement of (+) charges into the cell results in a small depolarization of the neuron, which is a graded potential only, referred to as an excitatory postsynaptic potential (EPSP).
- Too few Na+ channels are opened by a single action potential occurring at a single excitatory synapse to depolarize the membrane all the way to the threshold, but it brings the membrane potential closer to it.
- This increases the probability that subsequent stimuli will proceed to threshold depolarization and that the postsynaptic neuron will generate an action potential. The neurotransmitter’s binding to its receptor increases membrane permeability to K+ ions or Cl– ions through chemical messenger-gated channels at an inhibitory synapse.
- As a result, K+ ions can leave the cell down their gradient of concentration carrying (+) outward charges or Cl– ions can enter the cell down their gradient of concentration carrying(-) inward charges.
- In either case, relative to the outside, the neuron becomes more negative inside and the membrane is now hyperpolarized. As an inhibitory postsynaptic potential (IPSP), this small hyperpolarization is referred to.
- The movement of the membrane potential further away from the threshold decreases the probability that the postsynaptic neuron will produce an action potential.
- A neuron is almost invariably programmed genetically to synthesise and release only one type of neurotransmitter. A given synapse, therefore, is either always excitatory or always inhibitory.
- Once a neurotransmitter is attached to its postsynaptic neuron receptor and causes its effect, in order to prevent its continued activity indefinitely, it is important to inactivate or remove it from the synapse.
There have been several mechanisms identified to carry this out:
- Neurotransmitter passive diffusion away from the synaptic cleft.
- Destruction of the neurotransmitter by enzymes located in the synaptic cleft or presynaptic or postsynaptic neuron plasma membranes.
- Active reuptake of the neurotransmitter for reuse or enzymatic destruction into the synaptic knob of the presynaptic neuron.
Summation
- As previously mentioned, a single action potential at a single synapse results in a graded potential only: an EPSP or an IPSP. Therefore, generation of an action potential in the postsynaptic neuron requires the addition or summation of a sufficient number of excitatory inputs to depolarize this neuron to threshold. Two types of summation may occur:
- Temporal summation
- Spatial summation
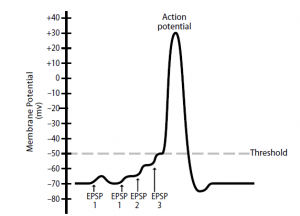
- Temporal summation occurs when multiple EPSPs (or IPSPs) produced in close sequence by a single presynaptic neuron exert an effect on the postsynaptic neuron’s membrane potential. An action potential in the presynaptic neuron, for example , produces an EPSP and partial postsynaptic neuron depolarization.
- While the postsynaptic neuron is still depolarized, in the postsynaptic neuron, a second action potential in the presynaptic neuron produces another EPSP that adds to the first and depolarizes this neuron further.
- As more EPSPs are added together, the membrane depolarizes until an action potential is generated closer to the threshold. Although the temporal summation is shown in Figure 2 with the summation of relatively few EPSPs, it may actually be necessary to add up to 50 EPSPs to reach the threshold.
- Since up to 500 action possibilities per minute can be generated by a presynaptic neuron, temporal summation occurs quite easily. Therefore, the strength of the signal to the postsynaptic neuron is affected by the presynaptic neuron’s frequency of nerve impulses generated.
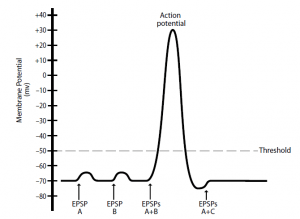
- When multiple EPSPs (or IPSPs), produced by many presynaptic neurons, exert their effects simultaneously on the membrane potential of the postsynaptic neuron, spatial summation happens. For instance, a single postsynaptic neuron that is innervated by three presynaptic neurons is represented in Figure 3.
- The presynaptic neuron A and B inputs are excitatory and the presynaptic neuron C input is inhibitory. Again, single action potentials in neuron A or B generate individual EPSPs that are insufficient to depolarize the threshold of the postsynaptic neuron.
- However, if EPSPs are produced at the same time from neurons A and B, the depolarizations add together and the threshold is reached by the membrane potential of the postsynaptic neuron, resulting in the generation of an action potential.
- Inputs from simultaneously occurring neurons A (excitatory) and C (inhibitory) may, in effect, cancel each other out, resulting in no change in the postsynaptic neuron’s membrane potential. This example, as with temporal summation, has been simplified to clearly illustrate the concept.
- In reality, to depolarize the postsynaptic neuron to the threshold, a large number of excitatory inputs from different presynaptic neurons are needed. Because thousands of presynaptic inputs are received by a typical neuronal cell body, spatial summation also happens quite readily.
- Therefore, the number of presynaptic neurons that are active simultaneously affects the strength of the postsynaptic neuron signal. Temporal summation and spatial summation may occur concurrently under normal physiological conditions.
Interconnections between neurons
- There are very extensive interconnections or communication between neurons in humans. Imagine the electrical activity complexity that can occur in the human brain among 100 billion neurons where each of these neurons provides input to and receives input from hundreds of other neurons.
- The diversity of these interconnections is responsible for the uniqueness of many abstract neurological phenomena in people, such as intellect, personality and memory. The two types of interconnections are:
- Convergence
- Divergence
- When the axon terminals of many presynaptic neurons all synapse with a single postsynaptic neuron, convergence occurs. Spatial summation of nerve impulses relies on the presence of convergence.
- When the axon of a single presynaptic neuron branches and synapses with multiple postsynaptic neurons, divergence takes place. Thus, activity in a single nerve fibre may affect several regions of the nervous system at the same time, each having a different function.
Factors affecting synaptic transmission
Several factors influence synaptic transmission of electrical impulses:
- pH of the interstitial fluid
- Hypoxia
- Drugs, toxins, and diseases
- Neurons are very sensitive to alterations in the pH of the surrounding interstitial fluid. The arterial blood pH is normally 7.4.
- The excitability of neurons also increases under conditions of alkalosis, in which pH increases, rendering them more likely to generate potential for action.
- This inappropriate stimulation of the nervous system, particularly in epileptics predisposed to them, can lead to seizures.
- The excitability of neurons is depressed under conditions of acidosis in which pH decreases, rendering them less likely to generate potential for action.
- A comatose state may result from this lack of stimulation of the nervous system. Coma is often caused by severe diabetic acidosis or acidosis associated with end-stage renal failure.
- Neuronal activity relies on a continuous supply of oxygen. Neuronal activity is depressed by hypoxia, a decrease in oxygen availability.
- Blood flow disruption to the brain for only a couple of seconds leads to unconsciousness. A prolonged lack of blood flow, which is characteristic of stroke, leads to permanent brain damage in the affected area.
- Many drugs, toxins, and diseases exert their clinical effects by altering some phase of synaptic activity. These effects may occur by means of:
- Altered release of a neurotransmitter
- Altered interaction of a neurotransmitter with its receptor
- Altered removal of a neurotransmitter from the synaptic cleft
- Replacement of a deficient neurotransmitter
Altered release.
- Tetanus is an infectious disease caused by Clostridium tetani, a bacterium. This bacterium produces a neurotoxin that is active in the spinal cord during inhibitory synapses.
- Motor neurons have cell bodies that lie in the spinal cord, which supply skeletal muscle and cause contraction. These motor neurons receive excitatory and inhibitory inputs from different sources under normal circumstances.
- The result of the balance of these inputs is the appropriate degree of muscle tone or contraction of the muscle. The release of gamma amino butyric acid ( GABA), an important neurotransmitter active in these inhibitory synapses, is prevented by the tetanus toxin.
- Eliminating inhibitory inputs results in excitatory input to the motor neurons being unchecked or unmodulated.
- In the muscles of the jaw, the resulting uncontrolled muscle spasms originally occur, giving rise to the lockjaw expression. Muscle spasms eventually affect the respiratory muscles, thus avoiding inspiration and leading to asphyxiation-related death.
Altered interaction of a neurotransmitter with its receptor.
- A neurotransmitter ‘s interaction with its receptor may be pharmacologically altered in several ways. One such mechanism involves the administration of antagonists, drugs that bind to a given receptor and prevent the neurotransmitter ‘s action but initiate no other effect by classical definition.
- Schizophrenia, a severe mental disorder characterised by delusions , hallucinations, social withdrawal, and disorganised speech and behaviour, is an interesting clinical example of this form of therapy.
- Although the precise cause of schizophrenia is unknown, neuronal pathways that release excessive amounts of the neurotransmitter dopamine appear to be involved in its pathophysiology.
- By blocking dopamine receptors and thus preventing excess dopamine from exerting its effects, antipsychotic drugs such as Thorazine ® (chlorpromazine) and Haldol ® (haloperidol) minimise symptoms of schizophrenia.
- A drug that binds to and stimulates a given receptor is an agonist. In other words, the effect of endogenous neurotransmitters is mimicked by agonists. Albuterol is a 2-adrenergic receptor agonist that mimics the effect of the neurotransmitter, epinephrine, the active ingredient in medications such as Ventolin ®.
- Since stimulation of these receptors in the lungs causes dilation of the airways, albuterol is effective in reversing asthma-related bronchospasm and dyspnea (difficulty breathing).
- The administration of drugs that facilitate the binding of the neurotransmitter to its receptor is another mechanism by which neurotransmitter / receptor interaction may be altered. Again, GABA, the most prevalent inhibitory neurotransmitter in the nervous system, is the neurotransmitter used as an example.
- It not only contributes to the regulation of skeletal muscle tone by inhibiting motor neuron activity, but also acts as a CNS depressant to regulate mood and emotions.
- The anti-anxiety drugs benzodiazepines, which include Valium ® (diazepam) and Ativan ® (lorazepam), act by binding to the GABA receptor at a specific site.
- This binding triggers a conformational change in the protein of the receptor that improves GABA binding. As more GABA binds to the receptors, it increases its efficiency in the CNS and decreases anxiety.
Altered removal of a neurotransmitter from the synaptic cleft.
- Changes in neurotransmitter reuptake or degradation are involved in the third mechanism by which drugs may alter synaptic activity.
- Prozac ® (fluoxetine), which is used for treating depression, is a very well-known example of a drug in this category.
- The complete aetiology is unknown, but depression is widely accepted to include a deficiency in the CNS of monoamine neurotransmitters (e.g., norepinephrine and serotonin).
- Serotonin removal from the synaptic cleft is prevented by Prozac, a selective serotonin reuptake inhibitor. As a result, serotonin concentration and activity are enhanced.
Replacement of a deficient neurotransmitter.
- Finally, replacement of a deficient neurotransmitter, a form of drug therapy effective in the treatment of Parkinson’s disease, may alter synaptic activity.
- Parkinson’s pathophysiology includes the progressive destruction of dopaminergic (dopamine-releasing) neurons, resulting in dopamine deficiency in certain brain areas.
- In addition to neuronal pathways involved in mood and emotion regulation, neurons that inhibit skeletal muscle contraction release dopamine.
- As motor neurons normally receive excitatory and inhibitory inputs, smooth, accurate muscle contractions result from the inhibition provided by the dopaminergic pathways.
- This loss of inhibition leads to increased muscle tone, or muscle rigidity, and resting tremors in patients with Parkinson’s disease.
- By administering levodopa (L-dopa), a precursor to dopamine, these symptoms are alleviated. The axon terminals of dopaminergic neurons are taken up by L-dopa and used to form dopamine.
- Interestingly, in some patients, the development of symptoms characteristic of schizophrenia is a side effect of dopamine replacement therapy. (Remember that overactive dopaminergic neurons cause this mental disorder.) On the other hand, drugs used to treat schizophrenia, dopamine receptor antagonists, may elicit Parkinson’s disease symptoms.
REFERENCES:
