Introduction to Cholesterol
- Definition: Cholesterol is a sterol, a type of lipid molecule with a characteristic four-ring structure, found in all animal cells.
- Chemical Structure: Comprises a hydrocarbon tail, a central sterol nucleus with four fused rings, and a hydroxyl group, making it an amphipathic molecule.
- Sources of Cholesterol:
- Endogenous: Synthesized primarily in the liver, intestines, and other tissues.
- Exogenous: Obtained from dietary sources like meat, eggs, and dairy products.
- Functions of Cholesterol:
- Structural Role: Integral component of cell membranes, maintaining fluidity and stability.
- Precursor Molecule: Serves as a precursor for steroid hormones (e.g., cortisol, testosterone), bile acids, and vitamin D.
- Signaling: Participates in cellular signaling pathways, including hedgehog signaling.
- Health Implications: Imbalanced cholesterol levels are linked to atherosclerosis, heart disease, and metabolic syndromes.
What is Cholesterol Metabolism?
- Cholesterol metabolism encompasses the complex biochemical processes involved in cholesterol synthesis, transport, utilization, and elimination
- Essential for maintaining cellular membrane integrity, hormone production, and bile acid synthesis
- Dysregulation leads to cardiovascular diseases, the leading cause of death globally
Why Cholesterol Biosynthesis Matters
- Body produces approximately 1-2 grams of cholesterol daily
- Dietary cholesterol contributes only 25% of total body cholesterol
- Endogenous synthesis is the primary source (75%) of cholesterol in healthy individuals
Importance of Cholesterol Metabolism
- Metabolic Balance: Cholesterol metabolism ensures a balance between synthesis, uptake, and excretion to maintain homeostasis.
- Energy Storage and Utilization: Cholesterol derivatives, like bile acids, aid in fat digestion and nutrient absorption.
- Disease Prevention: Proper regulation prevents excessive cholesterol accumulation, reducing the risk of cardiovascular diseases.
- Therapeutic Target: Understanding cholesterol metabolism is crucial for developing drugs like statins, which target cholesterol biosynthesis.
Cholesterol Biosynthesis
- Location: Primarily occurs in the liver’s cytoplasm and endoplasmic reticulum (ER).
- Key Enzyme: HMG-CoA reductase (3-hydroxy-3-methylglutaryl-coenzyme A reductase) is the rate-limiting enzyme.
- Starting Material: Acetyl-CoA, a product of carbohydrate, fat, and protein metabolism.
- End Product: Cholesterol, a 27-carbon molecule.
- Energy Requirement: Biosynthesis is energy-intensive, requiring ATP and NADPH.
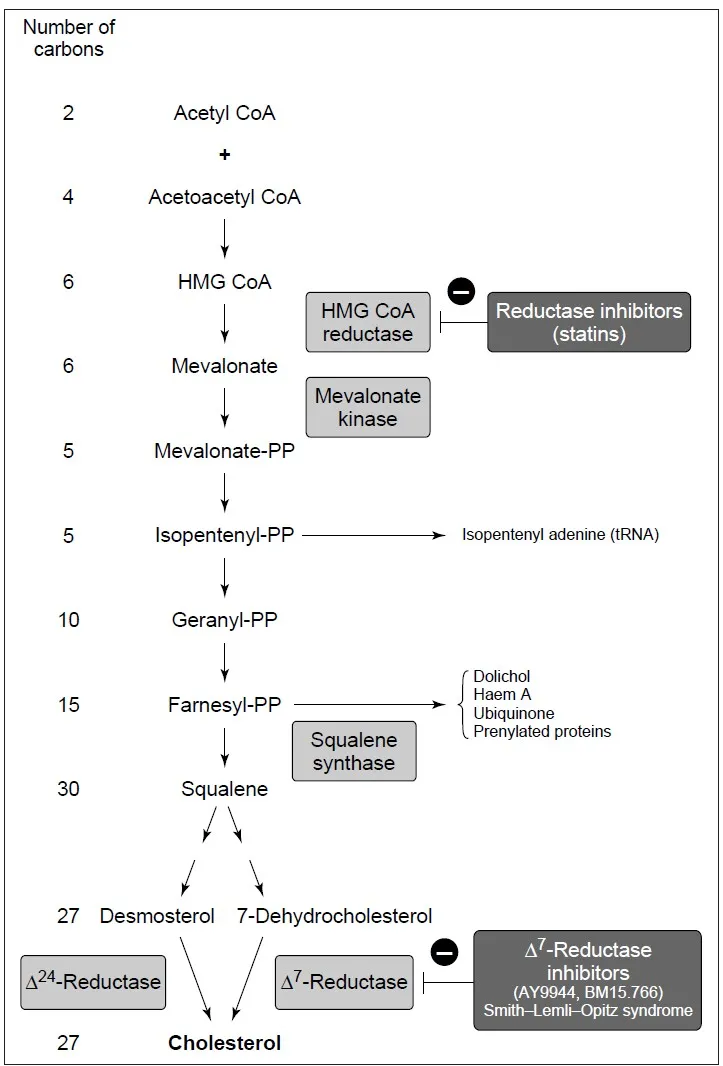
- Pathway: Known as the mevalonate pathway, it involves multiple enzymatic steps divided into three stages.
Detailed Steps of Cholesterol Biosynthesis
The biosynthesis of cholesterol occurs via the mevalonate pathway, which can be divided into three main stages:
- Formation of mevalonate
- Conversion of mevalonate to isoprenoid units
- Condensation of isoprenoid units to form cholesterol
Stage 1: Formation of Mevalonate
- Starting Point: Two molecules of acetyl-CoA combine to initiate the pathway.
- Key Steps:
- Condensation: Two acetyl-CoA molecules form acetoacetyl-CoA, catalyzed by thiolase.
- HMG-CoA Formation: Acetoacetyl-CoA reacts with a third acetyl-CoA molecule to form HMG-CoA, catalyzed by HMG-CoA synthase.
- Reduction to Mevalonate: HMG-CoA is reduced to mevalonate by HMG-CoA reductase, using two molecules of NADPH.
- Significance: HMG-CoA reductase is the primary regulatory enzyme, targeted by statins to lower cholesterol levels.
Table 1: Stage 1 of Cholesterol Biosynthesis
| Step | Enzyme | Substrate | Product | Cofactor |
|---|---|---|---|---|
| Condensation | Thiolase | 2 Acetyl-CoA | Acetoacetyl-CoA | None |
| HMG-CoA Formation | HMG-CoA Synthase | Acetoacetyl-CoA + Acetyl-CoA | HMG-CoA | None |
| Reduction | HMG-CoA Reductase | HMG-CoA | Mevalonate | 2 NADPH |
Stage 2: Conversion of Mevalonate to Isoprenoid Units
- Phosphorylation: Mevalonate is phosphorylated by mevalonate kinase and phosphomevalonate kinase to form mevalonate-5-pyrophosphate, requiring ATP.
- Decarboxylation: Mevalonate-5-pyrophosphate is converted to isopentenyl pyrophosphate (IPP) by mevalonate decarboxylase, releasing CO₂.
- Isomerization: IPP is isomerized to dimethylallyl pyrophosphate (DMAPP) by isopentenyl pyrophosphate isomerase.
- Significance: IPP and DMAPP are five-carbon isoprenoid units, the building blocks for cholesterol and other isoprenoids.
Table 2: Stage 2 of Cholesterol Biosynthesis
| Step | Enzyme | Substrate | Product | Cofactor |
|---|---|---|---|---|
| Phosphorylation 1 | Mevalonate Kinase | Mevalonate | Mevalonate-5-phosphate | ATP |
| Phosphorylation 2 | Phosphomevalonate Kinase | Mevalonate-5-phosphate | Mevalonate-5-pyrophosphate | ATP |
| Decarboxylation | Mevalonate Decarboxylase | Mevalonate-5-pyrophosphate | IPP | ATP |
| Isomerization | IPP Isomerase | IPP | DMAPP | None |
Stage 3: Condensation of Isoprenoid Units to Form Cholesterol
- Formation of Geranyl Pyrophosphate: IPP and DMAPP combine to form geranyl pyrophosphate (GPP), a 10-carbon molecule, catalyzed by geranyl pyrophosphate synthase.
- Formation of Farnesyl Pyrophosphate: GPP reacts with another IPP to form farnesyl pyrophosphate (FPP), a 15-carbon molecule, catalyzed by farnesyl pyrophosphate synthase.
- Squalene Synthesis: Two FPP molecules combine to form squalene, a 30-carbon molecule, catalyzed by squalene synthase.
- Cyclization: Squalene is oxidized to squalene-2,3-epoxide by squalene epoxidase, requiring O₂ and NADPH.
- Formation of Lanosterol: Squalene-2,3-epoxide cyclizes to form lanosterol, catalyzed by lanosterol synthase.
- Conversion to Cholesterol: Lanosterol undergoes multiple enzymatic modifications (e.g., demethylation, desaturation) to form cholesterol, involving enzymes like CYP51 and 7-dehydrocholesterol reductase.
Table 3: Stage 3 of Cholesterol Biosynthesis
| Step | Enzyme | Substrate | Product | Cofactor |
|---|---|---|---|---|
| GPP Formation | Geranyl Pyrophosphate Synthase | IPP + DMAPP | GPP | None |
| FPP Formation | Farnesyl Pyrophosphate Synthase | GPP + IPP | FPP | None |
| Squalene Synthesis | Squalene Synthase | 2 FPP | Squalene | NADPH |
| Epoxidation | Squalene Epoxidase | Squalene | Squalene-2,3-epoxide | O₂, NADPH |
| Cyclization | Lanosterol Synthase | Squalene-2,3-epoxide | Lanosterol | None |
| Final Modifications | Multiple Enzymes (e.g., CYP51) | Lanosterol | Cholesterol | Various |
Regulation of Cholesterol Biosynthesis
Cholesterol biosynthesis is tightly regulated to prevent overproduction or deficiency, ensuring cellular and systemic homeostasis. Regulation occurs at multiple levels: transcriptional, post-transcriptional, and post-translational.
1. Transcriptional Regulation
- Key Regulator: SREBP (Sterol Regulatory Element-Binding Protein), a transcription factor.
- Mechanism:
- Low Cholesterol Levels: When cellular cholesterol is low, SREBP is cleaved from the ER membrane by SCAP (SREBP cleavage-activating protein) and translocates to the nucleus.
- Gene Activation: SREBP activates genes encoding HMG-CoA reductase, HMG-CoA synthase, and other enzymes in the mevalonate pathway.
- High Cholesterol Levels: Excess cholesterol inhibits SREBP cleavage, reducing gene expression.
- Feedback Loop: Cholesterol and its derivatives (e.g., oxysterols) bind to INSIG (Insulin-Induced Gene) proteins, retaining SREBP-SCAP in the ER.
2. Post-Transcriptional Regulation
- mRNA Stability: HMG-CoA reductase mRNA stability is modulated by cellular sterol levels.
- MicroRNAs: Specific microRNAs (e.g., miR-33) target HMG-CoA reductase mRNA, reducing its translation under high cholesterol conditions.
3. Post-Translational Regulation
- Enzyme Degradation: HMG-CoA reductase is degraded via the ubiquitin-proteasome pathway when cholesterol levels are high.
- Phosphorylation: AMP-activated protein kinase (AMPK) phosphorylates HMG-CoA reductase, reducing its activity during energy stress.
- Allosteric Regulation: Mevalonate and other intermediates can inhibit HMG-CoA reductase activity.
4. Hormonal Regulation
- Insulin: Stimulates HMG-CoA reductase expression, promoting cholesterol synthesis.
- Glucagon: Inhibits cholesterol synthesis by reducing HMG-CoA reductase activity.
- Thyroid Hormones: Enhance SREBP expression, increasing cholesterol biosynthesis.
5. Dietary and Pharmacological Regulation
- Dietary Cholesterol: High dietary cholesterol suppresses endogenous synthesis via feedback inhibition.
- Statins: Competitive inhibitors of HMG-CoA reductase, reducing cholesterol production and upregulating LDL receptor expression.
Table 4: Regulatory Mechanisms of Cholesterol Biosynthesis
| Level | Mechanism | Key Players | Effect |
|---|---|---|---|
| Transcriptional | SREBP Activation | SREBP, SCAP, INSIG | Upregulates enzyme genes |
| Post-Transcriptional | mRNA Stability, MicroRNAs | miR-33 | Modulates HMG-CoA reductase expression |
| Post-Translational | Degradation, Phosphorylation | Ubiquitin-Proteasome, AMPK | Reduces enzyme activity |
| Hormonal | Insulin, Glucagon, Thyroid Hormones | SREBP, HMG-CoA Reductase | Stimulates or inhibits synthesis |
| Dietary/Pharmacological | Statins, Dietary Cholesterol | HMG-CoA Reductase, LDL Receptors | Inhibits synthesis |
Clinical Relevance of Cholesterol Metabolism
- Atherosclerosis: Excessive cholesterol accumulation in arterial walls leads to plaque formation, increasing cardiovascular risk.
- Dyslipidemia: Imbalances in cholesterol metabolism (e.g., high LDL, low HDL) are linked to heart disease.
- Statins: Widely used to lower LDL cholesterol by inhibiting HMG-CoA reductase, reducing cardiovascular events.
- Genetic Disorders:
- Familial Hypercholesterolemia: Mutations in LDL receptor genes impair cholesterol uptake, leading to elevated plasma cholesterol.
- Smith-Lemli-Opitz Syndrome: Deficiency in 7-dehydrocholesterol reductase disrupts cholesterol synthesis, causing developmental abnormalities.
- Therapeutic Advances: PCSK9 inhibitors enhance LDL receptor recycling, further lowering cholesterol levels.
Frequently Asked Questions
- What is the primary enzyme in cholesterol biosynthesis?
- HMG-CoA reductase is the rate-limiting enzyme, targeted by statins to reduce cholesterol levels.
- How does the body regulate cholesterol levels?
- Through transcriptional (SREBP), post-transcriptional (microRNAs), post-translational (degradation, phosphorylation), and hormonal mechanisms.
- What are the health risks of dysregulated cholesterol metabolism?
- It can lead to atherosclerosis, heart disease, and genetic disorders like familial hypercholesterolemia.
- How do statins work?
- Statins inhibit HMG-CoA reductase, reducing cholesterol synthesis and increasing LDL receptor expression.
- Can diet affect cholesterol biosynthesis?
- Yes, high dietary cholesterol suppresses endogenous synthesis via feedback inhibition.
References
- Brown, M. S., & Goldstein, J. L. (1997). The SREBP pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell, 89(3), 331-340. Link
- Horton, J. D., Goldstein, J. L., & Brown, M. S. (2002). SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. Journal of Clinical Investigation, 109(9), 1125-1131. Link
- Russell, D. W. (2003). The enzymes, regulation, and genetics of bile acid synthesis. Annual Review of Biochemistry, 72, 137-174. Link
- National Heart, Lung, and Blood Institute. (2023). Cholesterol Management. Link
- Endo, A. (2010). A historical perspective on the discovery of statins. Proceedings of the Japan Academy, Series B, 86(5), 484-493. Link
