Major pathways of Intracellular Cell Signalling
Upon binding to ligand, most cell surface receptors stimulate intracellular target enzymes to transmit and amplify the signal. An amplified signal may be propagated to the nucleus to regulate gene expression in response to an external cell stimulus. Major intracellular signalling pathways include the cAMP and cGMP pathways, the C-Ca2 + phospholipase pathway, the NF-kB (for nuclear factor involved in the transcription of the k light chain gene in b lymphocytes) transcription factor pathway, the Ca2 + -calmododulin pathway, the MAP (for mitogen-activated protein) kinase pathway, and the JAK-STAT (for signal transducers and transc actuators).
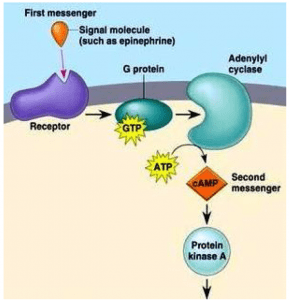
- The cAMP pathway
In 1958, the cAMP-mediated intracellular signalling pathway was discovered by Earl Sutherland while studying the action of epinephrine, a hormone that breaks glycogen into glucose before muscle contraction. When epinephrine binds to its receptor, the intracellular concentration of cAMP is increased. CAMP is formed from adenosine triphosphate ( ATP) by the action of the enzyme adenyl cyclase and degraded to adenosine monophosphate (AMP) by the enzyme cAMP phosphodiesterase. This mechanism led to the concept of the first messenger mediating the cell-signalling effect of the second messenger, CAMP. The epincphrine receptor is associated with G-protein adenyl cyclase, which stimulates the ativity of the cyclase on epinephrine binding. The intracellular signalling effects of cAMP are mediated by the enzyme cAMP-dependent protein kinase (or protein kinase A). In its inactive form, protein kinase A is a tetramer consisting of two regulatory subunits (to which cAMP-binds) and two catalytic subunits. Binding the cAMP results in the dissociation of the catalytic subunits. Free catalytic subunits may phosphorylate serine residues on target proteins. In the epinephrine-dependent regulation of the metabolism of glycogen, protein kinase
A phosphorylates two enzymes:
- Phosphorylase kinase, which in turn phosphorylates glycogen phosphorylase to break down glycogen into glucose-I-phosphate.
- Glycogen synthase, which is involved in the synthesis of glycogen. Phosphorylation of glycogen synthase prevents the synthesis of glycogen.
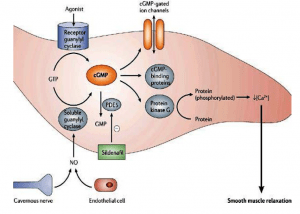
- The cGMP pathway
CGMP is a second messenger, too. It is produced by guanylate cyclase from guanosinetriphosphate (GTP) and degraded to GMP by phosphodiesterase. Guanylate cyclases are activated by nitric oxide and peptide signalling molecules. The best characterised role of cGMP is in the photoreceptor rod cells of the retina, where light signals are converted to nerve impulses.
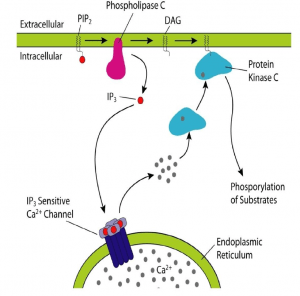
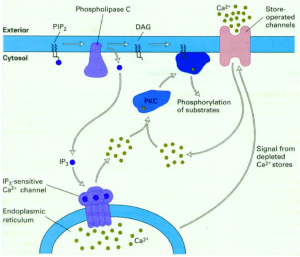
- Phospholipase C-Ca2+ pathway
A second messenger involved in intracellular signalling is derived from phospholipid phosphatidylinositol 4.5-bisphosphate (PIP2) present in the inner leaflet of the plasma membrane. The enzyme phospholipase C (PLC) hydrolysis of PIP2-stimulated by a number of hormones and growth factors-produced two second messengers: diacylglycerol and inositol 1,4,5-trisphosphate (IP3). These two messengers stimulate two downstream signalling cascades: protein kinase C and Ca2 + mobilisation. There are two forms of PLC: PLC-ß and PLC-ɣ. PLC-ß is activated by protein G. PLC-ɣ contains SH2 domains that enable tyrosine kinase receptor protein association. Tyrosine phosphorylation increases PLC-ɣ activity, which in turn stimulates the breakdown of PIP2. Diacylglycerol, derived from PIP2 hydorlysis, activates members of the protein kinase C family (protein serine and thronene kinase). Phorbol esters are tumour growth-promoting agents that act, such as diacylglycerol, by stimulating protein kinase C activity. Protein kinase C activates other intracellular targets such as MAP kinase protein kinase pathways to produce transcription phosphorylation factors leading to changes in gene expression and cell proliferation.
- NF-kB transcription factor pathway
NF-kB is a transcription factor involved in immune responses in several cells and is stimulated by protein kinase C In its inactive state, the NF-kB and the complex are retained in the cytoplasm. I-kB phosphorylation – triggered by I-kB kinase – leads to the destruction of I-kB by the 26S proteasome and the release of NF-kB. The free NF-kB heterodimer is transferred to the nucleus to activate gene transcription in response to immunological and inflammatory signalling.
- Ca2+- calmodulin pathway
Although the second messenger diacylglycerlol remains associated with the plasma membrane, the second messenger IP3 derived from PIP2 is released into the cytosol to activate ion pumps and free Ca2 + from intracellular storage sites. High concentrations of Cytosolic Ca2 + (from a basal level of 0.1 UM to an increased concentration of 1.0 uM after cytosolic release) activate several Ca2 + -dependent protein kinases and phosphatases. Calmodulin is a Ca2 + -dependent protein that is activated when the concentration of Ca2 + increases to 0.5 uM. Ca2 + -calmodulin complexes bind to a number of cytosolic target proteins to regulate cell reactions. Note that Ca2 + is an important second messenger and that its intracellular concentration can be increased not only by releasing Ca2 + from intracellular storage sites, but also by increasing Ca2 + entry from extracellular space into the cell.
- MAP kinase pathway
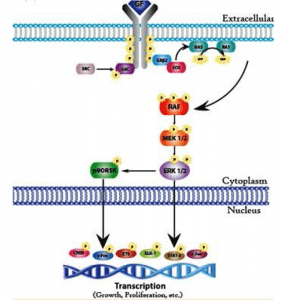
This pathway involves evolutionarily conserved protein kinases (yeast to humans) with roles in cell growth and differentiation. MAP kinases are protein serin and threonine kinases that are activated by growth factors and other signalling molecules. The ERK family is a well-defined form of MAP kinase. Members of the ERK family (for extracellular signal-controlled kinase) act by either protein tyrosine kinase or protein G — associated receptors. Both CAMP and Ca2 + -dependent pathways can stimulate or inhibit ERK pathways in different cell types.
Activation of ERK is mediated by two protein kinases: raf, a serine protein or threonine kinase, which in turn activates a second kinase called MEK (for MAP kinase or ERK kinase). Stimulation of the growth factor receptor leads to activation of the GTP binding protein Ras (for rat sarcoma virus) that interacts with Raf. Raf phosphorylates and activates MEK, which then activates ERK by phosphorylation of the serine and threonine residue. ERK then phosphorylates the target nuclear and cytosolic proteins. Activated ERK phosphorylates transcription factors EIK-I (for E-26-like protein I) and serum response factor (SRF) in the nucleus. Recognizing the regulatory sequence called the Serum Response Element (SRE).
In addition to ERK, mammalian cells contain two additional MAfP kinases called JNK and p38 MAP kinases. Cytokines and ultraviolet irradiation stimulate JNK and p38 MAP kinase activation mediated by small GTP binding proteins different from Ras. These kinases are not activated by MEK but by a distinct dual kinase called MKK (for MAP kinase). Ras proteins, a group of tumour virus oncogenic proteins that cause sarcomas in rats, are a key component of the ERK pathway. Mutations of the Ras gene have been linked to human cancer. Ras proteins are guanine nucleotide-binding proteins with functional properties similar to G protein A subunits (activated by GTP and inactivated by guanosine diphosphate[GDP]). The difference with G protein is that Ras proteins are not associated with subunits. Ras is activated by the exchange factors for guanine nucleotide to facilitate the release of GDP in exchange for GTP. The activity of the Ras GTP complex is terminated by GTP hydrolysis, stimulated by GTPase-activating proteins. In human cancers, mutation f Ras genes results in a breakdown of GTP and therefore the mutated Ras protein remains continuously in the active GRP-bound form.
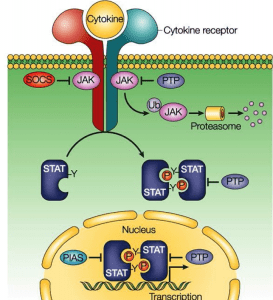
- JAK-STAT pathway
The preceding MAP kinase pathway links the cell surface to the nucleus signalling mediated by a protein kinase cascade leading to phosphorylation of transcription factors. The JAK-STAT pathway provides a close link between protein tyrosine kinases and transcription factors by directly affecting transcription factors. STAT (for signal transducers and transcription activators) proteins are transcription factors in the SH2 domain and are present in an inactive state in the cytoplasm. Ligand binding receptor stimulation recruits STAT proteins that bind to the cytoplasmic protion of the receptor associated with JAK protein tyrosine kinase through their SH2 domain and become phosphorylated. Phosphorylated STAT proteins then digest and translocate into the nucleus where they activate the transcription of the target genes.