Osmoregulation
Osmoregulation is concerned with the regulation of body fluid solute concentration in the animal and the maintenance of water balance.
Body fluid Compartments
Most animals comprise mainly water: a 60 kg adult man contains about 40 liters of water, 25 liters within cells and the rest in extracellular fluids. The extracellular body fluids include:
- Interstitial water between cells
- Body cavity fluids (e.g. in coelom)
- Blood
- Lymph
The extracellular body fluids need to remain in osmotic balance with the fluid in cells from which they are separated by the cell membrane. This may include adjustment of electrolyte concentrations or organic solute concentrations (which may differ on each side of the membrane: intracellular fluid has a higher potassium concentration than extracellular fluid, whereas the reverse applies for sodium).
It is important that such differences are maintained, particularly in excitable cells such as neurons. Extracellular fluids are routes for the transport of gas, food, waste, etc. to and from cells. The body fluids need to be in osmotic balance with external fluids in the case of aquatic animals, or some means of control must be supplied: this may be critical in cells closest to the external environment (as in the respiratory, urino-genital and alimentary tracts).
Extracellular fluid
Total solute concentration determines the osmotic pressure of the solution. Cell membranes act as semi-permeable membranes, and increases in the total solute concentration of extracellular fluids may result in damaging withdrawal of water from cells.
Sodium (Na+) is the main cation and chloride (Cl–) is the main anion, and together they are two of the main contributors to the fluids’ osmotic pressure; changes in Na+ and Cl– content promote water retention or excretion in order to maintain the osmotic pressure of the extracellular fluids. They are, therefore, key components in maintenance of fluid and thus of body volume.
Potassium (K+) is found principally within cells; fluctuation in extracellular K+ affects the electrical properties of nerve and muscle cell membranes.
Calcium (Ca2+) is critical in determining the excitability of nerve and muscle cells; about 50% of the plasma calcium is bound to plasma proteins, the rest is ionized in solution and is under close control.
Proton (H+) concentration (measurable as pH) is important in acid–base regulation in the body; the main sources of protons are from amino acid metabolism and from carbon dioxide.
![]()
Excess base (e.g. HCO3–) can combine with protons to remove them from solution. The high reactivity of protons renders it imperative that they be regulated in all body fluid compartments. Buffers such as hemoglobin and phosphate in cells, and bicarbonate/carbonic acid outside cells fulfill this role. Blood plasma pH is about 7.35–7.45; intracellular pH varies between 6 and 7 (due to acid metabolites in the cells). Protons are also excreted in the urine: thus the proton concentration in extracellular fluids is kept constant. (In some lung disorders where carbon dioxide is retained, there is a build-up of plasma proton levels as more HCO3– is generated; protons are removed by the kidneys and retention of bicarbonate ions by the kidneys increases the buffering capacity of the blood.)
Voiding of water can be also used secondarily to void waste products, or can be a primary method of voiding those products. Thus osmoregulatory and excretory organs are often combined. Regulation of the composition and volume of body fluids may include consideration of the following factors.
- The salt content of the environment in which an aquatic animal lives, and the necessity to maintain an internal salt balance. In fresh water, water will tend to flow into the animal by osmosis and expulsion of excess water will result in loss of salts; in salt water, water may flow out of the animal and compensatory drinking of salty water may result in salt overload.
- In terrestrial species there may be a tendency to desiccate in dry air.
- The water requirements of physiological processes (e.g. digestion, sweating).
- The need to eliminate nitrogenous wastes (which will require water which may be in short supply).
Osmoregulation and the environment
- Animals live in the sea, in brackish water, in fresh water and on land: all pose osmoregulatory problems. Seventy one percent of the earth’s surface is covered with water, 1% is fresh water (0.01% volume of sea water).
- The sea is 3.5% salt, the major ions being Cl–, Na+; additionally Mg2+, SO42– and Ca2+ are present in substantial amounts. (There are geographic variations: the Mediterranean has 4% salts, coasts and estuaries may be less saline, although relative proportions of ions stay approximately the same.)
- Some inland waters may be very saline: the Great Salt Lake, Utah, USA is saturated with NaCl: there are no fishes in this lake but the brine-shrimp Artemia sp. survives. The Dead Sea in the Jordan Rift Valley is saturated with several ions, and CaSO4 crystallizes out: only microorganisms survive.
- Brackish waters near estuaries are interesting as a transition site between freshwater and saltwater life forms. Fresh waters are very varied with respect to their solute content: droplets of ocean spray evaporate, and salt particles can be carried far inland and deposited with rain; leaching of salts from rocks occurs (e.g. Ca2+ from limestone).
Animals can be classed as:
- Euryhaline: tolerate wide salinity variation.
- Stenohaline: intolerant of wide salinity variation.
Aquatic Invertebrates
Most marine invertebrates are isosmotic: as osmoconformers they change their body fluid osmolarity to match the concentration of the medium; the concentration of solutes may differ from the medium, even if the osmolarity is the same (because of organic solvents); therefore, there may be ionic regulation. The osmoconforming jellyfish Aurelia sp. regulates to give low SO42– levels in its body fluids, giving it positive buoyancy. Mg2+ (which anesthetizes neuromuscular transmission) is down-regulated in fast-moving crab species.
Osmoregulators maintain the osmolarity of body fluids despite changes in the medium: many marine crabs in brackish water maintain a high body fluid osmolarity. Osmoregulators survive better in brackish water than do osmoconformers, although some are intermediate. The mitten crab Eriocheir sp. Can survive fresh water but returns to the sea to breed; Carcinus sp. can only survive if salts are at 33% of the sea water concentration. The brine-shrimp Artemia sp. is normally hyposmotic to its medium: salts from food are removed from the gut by active uptake and then eliminated by active transport across the gill epithelia.
All freshwater invertebrates are hyperosmotic to the medium and osmoregulate, although there are differences between concentrations of body fluids, that of a crayfish, Astacus sp., being five times that of a freshwater clam, Anodonta sp. Such freshwater animals face problems of osmotic inflow of water, especially through respiratory organs, and also through the gut. Excess water can be voided as dilute urine, but no animal is known which can void pure water so there will be a loss of salts. These can be compensated for by salts in food or by direct uptake from the medium, for example through the gills in cray-fishes by active transport processes.
Aquatic vertebrates
Aquatic vertebrates can be:
- Isosmotic (or slightly hyperosmotic) with respect to sea water (e.g. hagfishes, elasmobranchs, lungfishes, Rana cancrivora).
- Hyposmotic with respect to sea water (e.g. lampreys, teleosts). In fresh water all vertebrates are hyperosmotic.
Hagfishes (one of the two types of living jawless vertebrates) are marine and stenohaline: that is their salt concentration is equal to that of sea water. All other vertebrates keep their salts at a concentration lower than that of the sea.
Elasmobranchs (sharks and rays) are almost all marine. They are in osmotic equilibrium with the sea by adding urea and trimethylamine oxide (TMAO) to their body fluids; the shark kidney resorbs urea. Their plasma urea concentration is 100 times that of mammals. TMAO helps protect proteins from denaturation by urea.
Excess salts are excreted into the gut by a rectal gland. (There are a few freshwater elasmobranchs which all have low plasma urea concentrations, suggesting that this system is labile in evolution.) Holocephali (ratfishes) and the coelacanth also have high plasma urea and TMAO levels, using a similar osmoregulatory strategy to elasmobranchs.
Dipnoi (lungfishes) are freshwater forms which normally void ammonia via the gills; estivating dipnoans retain urea (to a concentration of 4%) in the blood, although this is associated more with estivation rather than being osmoregulatory.
The only sea-going amphibian, Rana cancrivora, the crab-eating frog from brackish mangrove swamps in the Mekong delta, also retains urea in its blood, giving it a blood slightly hyperosmotic to the sea: thus water flows in slowly through the skin, giving water for urine and obviating the need to drink salty water; the tadpole osmoregulates like a teleost, but the frog does need to seek fresh water to lay its eggs and for the period of metamorphosis. Freshwater amphibians maintain their plasma concentrations at about one-third that of sea water. Osmotic inflow of water is compensated for by voiding dilute urine: salt loss is restored by active transport of ions through the skin.
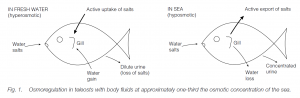
Teleosts, both marine and freshwater, are hyposmotic to the sea (approximately one-third of the sea water concentration).
- In fresh water they gain water through their gills and lose water by voiding dilute urine. Loss of salts in urine is compensated for by active uptake of ions through the chloride cells of the gills .
- In the sea they lose water via their gills and compensate by drinking. Excess salt is removed actively from the gut and is then excreted by active transport through the chloride cells in gills .
Skin in marine teleosts is very permeable to ions while skin in freshwater teleosts is much less permeable. The killifish adapts to both environments. The permeability of skin to ions drops in a few minutes when they are placed in fresh water, but recovery of permeability when they are placed in sea water takes some hours. Anadromous fishes (e.g. salmon) can live in both sea and fresh water. The osmotic flow of water can reverse, and active ion exchange at the gills can change direction; two separate populations of chloride cells are probably involved.
Terrestrial animals
Moist-skinned animals tend to desiccate in air: the dryer the air the greater the saturation deficit (i.e. the difference in vapor pressure of water in air over a free water surface and vapor pressure in air) and the more likely the animal is to dry out. The process is exacerbated by air movement (wind). Thus, moist skinned animals tend to live in moist microhabitats (earthworms, newts, frogs), and/or have shells in which to retreat (snails), and/or have protective mucus (slugs). Water loss is compensated for by drinking.
The Australian desert toad Chiroleptes platycephalus estivates in burrows several feet deep: very dilute urine is stored in the bladder. After rain, it surfaces and lays eggs which rapidly hatch, develop and metamorphose in transient puddles. Lungfishes are similar: in drought they make a coccoon with a breathing duct to the surface; they then estivate – protein metabolism results in high plasma urea concentrations. The desert snail Sphincterochila sp. Withdraws into its shell and secretes a calcareous epiphragm over the entrance, allowing it to survive mid-summer sunshine; during such a period of estivation water loss can be less than 500 μg per day (total water = 1.5 g).
Animals with waterproof skins do not face some of the difficulties encountered by moist-skinned forms; however, respiratory organs are often a source of water loss. Most terrestrial crustaceans need to return to water to lay eggs: Geocarcinus sp. can take up water from moist sand. Land woodlice need to dip their gill-books in water to keep them moist.
Insects and arachnids are very successful on land: the tracheae are lined with chitin and only the terminal tracheoles are water-permeable; ‘cyclic’ respiration whereby spiracles only open for brief periods while carbon dioxide is ‘blown off’ from the tissue fluids also conserves water in many larger insects, e.g. Locusta sp. In some species the cuticle can take up water. Damage to the waterproof wax coat of the cuticle can lead to a fatal dehydration. Many insects are very effective at resorbing water from urine and from feces; storage of excretory products (e.g. crystals of uric acid in the fat body) also conserves water. Metabolic water (produced during the oxidation of foodstuffs) can be utilized, and a few insects seem to be able to absorb water from air (e.g. the flea Xenopsylla sp. can absorb water from air with a relative humidity of at least 50%).
Reptiles possess thick, scaly, waterproof skins. Many desert species can tolerate fluctuations in blood electrolyte concentrations; for example Trachysaurus sp. can tolerate a blood sodium concentration of 150% normal. Marine reptiles such as the Galapagos marine iguana, crocodiles or sea-snakes drink sea water. Urine is hypotonic to the sea, so excess salts are excreted from nasal salt glands. Those feeding on teleosts ingest food which is hypotonic to sea water (see above). All birds have paired nasal salt glands too: in marine birds the salt glands are enlarged and secrete a hyperosmotic NaCl solution intermittently.
Marine mammals drink sea water, but their possession of kidneys with very long loops of Henle means that they can produce urine hypertonic to sea water. Thus there is a net gain of water to the seal or whale when it drinks sea water, but a human who drinks sea water becomes dehydrated:
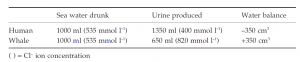
Such hypertonic urine and long kidney loops are also used as a water conservation ploy by desert mammals (e.g. jerboa); many of these mammals are not normally seen to drink water and rely on metabolic water gained from oxidation of foodstuffs.
