Amino Acids- A functional Group
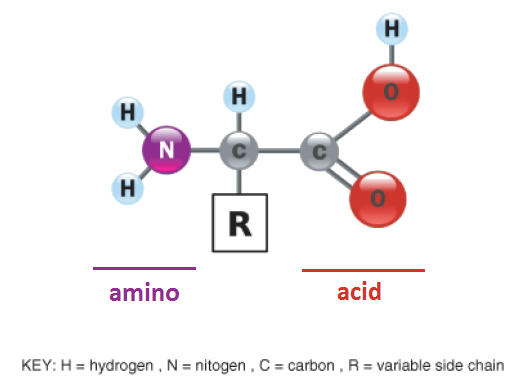
- Amino acids are a group of organic compounds containing two functional groups- amino and carboxyl.
- The amino group (-NH2) is basic while the carboxyl group (COOH) is acidic in nature.
- The general structure of amino acids The amino acids are termed as a-amino acids.
- If both the carboxyl and amino groups are attached to the same carbon atom, as depicted below The a-carbon atom binds to a side chain
- represented by R which is different for each of the 20 amino acids found in proteins.
- The amino acids mostly exist in the ionized form in the biological system.
- Optical isomers of amino acids If a carbon atom is attached to four different groups, it is asymmetric and therefore exhibits optical isomerism.
- The amino acids (except glycine) possess four distinct groups (R, H, COO, NH) held by o-carbon.
- Thus all the amino acids (except glycine where R = H) have optical isomers.
- The structures of L- and D-amino acids are written based on the configuration of L- and D-glyceraldehyde.
- The proteins are composed of L-a-amino acids
CLASSIFICATION OF AMINO ACIDS
- There are different ways of classifying the amino acids based on the structure and chemical nature, nutritional requirement, metabolic fate etc.
Amino acid classification based on the structure :
- A comprehensive classification of amino acids is based on their structure and chemical nature. Each amino acid is assigned a 3 letter or 1 letter symbol.
- These symbols are commonly used to represent the amino acids in protein structure.
- The 20 amino acids found in proteins are divided into seven distinct groups.
- Amino acids with aliphatic side chains :
- These are monoamino monocarboxylic acids. This group consists of the most simple amino acids glycine, alanine, valine, leucine and isoleucine.
- The last three amino acids (Leu, lle, Val) contain branched aliphatic side chains, hence they are referred to as branched-chain amino acids.
- Hydroxyl group-containing amino acids :
- Serine, threonine and tyrosine are hydroxyl group-containing amino acids.
- Tyrosine-being aromatic in nature—is usually considered under aromatic amino acids.
- Sulfur-containing amino acids :
- Cysteine with sulfhydryl group and methionine with thioester group are the two amino acids incorporated during protein synthesis.
- Cystine, another important sulfur-containing amino acid, is formed by condensation of two molecules of cysteine.
- Acidic amino acids and their amides :
- Aspartic acid and glutamic acids are dicarboxylic monoamine acids while asparagine and glutamine are their respective amide derivatives.
- All these four amino acids possess distinct codons for their incorporation into proteins.
- Basic amino acids :
- The three amino acids lysine, arginine (with guanidine group) and histidine (with imidazole ring) are dibasic monocarboxylic acids. They are highly basic in character.
- Aromatic amino acids :
- Phenylalanine, tyrosine and tryptophan (with indole ring)are aromatic amino acids. Besides these, histidine may also be considered under this category.
- Imino acids :
- Proline containing pyrrolidine ring is a unique amino acid. It has an amino group (=NH), instead of an amino group (-NH2) found in other amino acids. Therefore, proline is an a-amino acid.
- Classification of amino acids based on polarity: Amino acids are classified into 4 groups based on their polarity.
- The polarity, in turn, reflects the functional role of amino acids in protein structure.
- Non-polar amino acids: These amino acids are also referred to as hydrophobic (water-hating).
- They have no charge on the ‘R’ group. The amino acids included in this group are — alanine, leucine, isoleucine, valine, methionine, phenylalanine, tryptophan and proline.
- Polar amino acids with no charge on the ‘R’ group: These amino acids, as such, carry no charge on the ‘R’ group.
- They, however, possess groups such as hydroxyl, sulfhydryl and amide and participate in hydrogen bonding of protein structure.
- The simple amino acid glycine (where R= H) is also considered in this category.
- The amino acids in this group are- glycine, serine, threonine, cysteine, glutamine, asparagine and tyrosine.
- Polar amino acids with positive ‘R’ group: The three amino acids lysine, arginine and histidine are included in this group.
Polar amino acids with negative ‘R’ group :
- The dicarboxylic monoamine acids aspartic acid and glutamic acid are considered in this group.
Nutritional classification of amino acids :
- The twenty amino acids are required for the synthesis of a variety of proteins, besides other biological functions.
- However, all these 20 amino acids need not be taken in the diet.
- Based on the nutritional requirements, amino acids are grouped into two classes essential and non-essential.
Essential or indispensable amino acids :
- The amino acids cannot be synthesized by the body and, therefore,
- They need to be supplied through the diet are called essential amino acids.
- They are required for the proper growth and maintenance of the individual.
- The ten amino acids listed below are essential for humans (and also rats): Arginine, Valine, Histidine, Isoleucine, Leucine, Lysine, Methionine, phenylalanine, Threonine, Tryptophan.
- The two amino acids namely arginine and histidine can be synthesized by adults and not by growing children, hence these are considered as semi-essential amino acids (remember Ah, to recall).
- Thus, 8 amino acids are essential while semi-essential.
- Non-essential or dispensable amino acids: The body can synthesize about 10 amino acids to meet the biological needs,
- hence they need not be consumed in the diet. These are-glycine, alanine, serine, cysteine, aspartate, asparagine, glutamate, glutamine, tyrosine and proline.
PROPERTIES OF AMINO ACIDS
- The amino acids differ in their physicochemical properties which ultimately determine the characteristics of proteins.
PHYSICAL PROPERTIES
- Solubility: Most of the amino acids are usually soluble in water and insoluble in organic solvents.
- Melting points: Amino acids generally melt at higher temperatures, often above 200°C.
- Taste: Amino acids may be sweet (Gly, Ala, Val), tasteless (Leu) or bitter (Arg, lle).
- Monosodium glutamate (MSG; Ajinomoto) is used as a flavouring agent in the food industry, and Chinese foods to increase taste and flavour.
- In some individuals intolerant to MSG, Chinese restaurant syndrome (brief and reversible flu-like symptoms) is observed.
- Optical properties: All the amino acids except glycine possess optical isomers due to the presence of asymmetric carbon atom.
- Some amino acids also have a second asymmetric- carbon e.g. isoleucine, threonine. The structure 5 of L- and D-amino acids in comparison with glyceraldehyde.
- Amino acids as ampholytes: Amino acids contain both acidic (-COOH) and basic (-NH2) groups.
- They can donate a proton or accept a proton, hence amino acids are regarded as ampholytes.
- Zwitterion or dipolar ion: The name zwitter is derived from the German word which means hybrid.
- Zwitter ion (or dipolar ion) is a hybrid molecule containing positive and negative ionic groups. The amino acids rarely exist in a neutral form.
CHEMICAL PROPERTIES
- The general reactions of amino acids are mostly due to the presence of two functions.
- The amino groups behave as bases and combine with acids (e.g. HCI) to form salts (-NHZCH.
- Reaction with ninhydrin: The a-amino acids react with ninhydrin to form a purple, blue or pink colour complex (Ruheman’s purple).
- Colour reactions of amino acids: Amino acids can be identified by specific colour reactions.
- Transamination: Transfer of an amino. The group from an amino acid to a keto acid to form a new amino acid is a very important reaction in amino acid metabolism
- Oxidative deamination: The amino acids undergo oxidative deamination to liberate free ammonia.
References
https://www.ncbi.nlm.nih.gov/books/NBK234922/ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3042786/
