Contents:
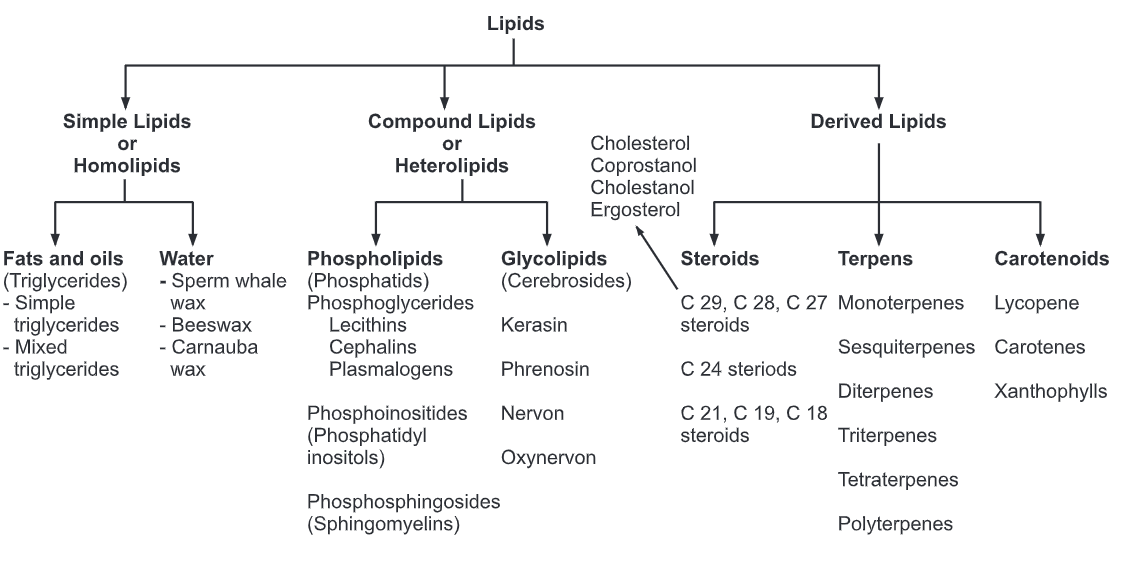
Classification of Lipids
Introduction
- Lipids are made of the elements Carbon, Hydrogen and Oxygen, but have a much lower proportion of water than other molecules such as carbohydrates.
- Unlike polysaccharides and proteins, lipids are not polymers, they lack a repeating monomeric unit.
- They are made up of two molecules: Glycerol and Fatty acids.
- A glycerol molecule is made up of three carbon atoms with a hydroxyl group attached to it and hydrogen atoms occupying the remaining positions.
- Fatty acids consist of an acid group at one end of the molecule and a hydrocarbon chain, which is usually denoted by the letter ‘R’.
- They may be saturated or unsaturated.
- A fatty acid is saturated if every possible bond is made with a Hydrogen atom, such that there exist no C=C bonds.
- Saturated fatty acids on the other hand contain C=C bonds. Monounsaturated fatty acids have one C=C bond, and polyunsaturated have more than one C=C bond.
- Lipids include fats, oils, waxes, and related compounds.
- They are widely distributed in nature both in plants and in animals.
Biological Importance of Lipids:
1. They are more palatable and storable to unlimited amount compared to carbohydrates.
2. They have a high-energy value (25% of body needs) and they provide more energy per gram than carbohydrates and proteins but carbohydrates are the preferable source of energy.
3. Supply the essential fatty acids that cannot be synthesized by the body.
4. Supply the body with fat-soluble vitamins (A, D, E, and K).
5. They are important constituents of the nervous system.
Tissue fat is an essential constituent of the cell membrane and nervous system. It is mainly phospholipids in nature that are not affected by starvation.
Lipids can be classified according to their hydrolysis products and according to similarities in their molecular structures.
Three major subclasses are recognized:
Simple Lipids
(a) Triglycerides
(b) Fats and oils which yield fatty acids and glycerol upon hydrolysis.
(c) Waxes, which yield fatty acids and long-chain alcohols upon hydrolysis.
(a) Triglycerides:
- They are uncharged due to the absence of ionizable groups in it.
- They are the most abundant lipids in nature. They constitute about 98% of the lipids of adipose tissue, 30% of plasma or liver lipids, less than 10% of erythrocyte lipids.
- They are esters of glycerol with various fatty acids. Since the three hydroxyl groups of glycerol are esterified, the neutral fats are also called “Triglycerides”.
- Esterification of glycerol with one molecule of fatty acid gives monoglyceride, and that with two molecules gives diglyceride.
1) Simple triglycerides: If the three fatty acids connected to glycerol are of the same type the triglyceride is called simple triglyceride, e.g., tripalmitin.
2) Mixed triglycerides: If they are of different types, called mixed triglycerides, e.g., stearo-diolein and palmito-oleo-stearin.
- A triglyceride (TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids. Triglycerides are a blood lipid that enable the bidirectional transfer of adipose fat and blood glucose from the liver. There are many triglycerides: depending on the oil source, some are highly unsaturated and some less.
(b) Fats and Oils:
- Both types of compounds are called triacylglycerols because they are esters composed of three fatty acids joined to glycerol, trihydroxy alcohol.
- The difference is on the basis of their physical states at room temperature. It is customary to call a lipid and fat if it is solid at 25°C and oil if it is a liquid at the same temperature.
- These differences in melting points reflect differences in the degree of unsaturation of the constituent fatty acids.
(c) Waxes:
- Wax is an ester of long-chain alcohol (usually mono-hydroxy) and a fatty acid.
- The acids and alcohols normally found in waxes have chains of the order of 12-34 carbon atoms in length.
Compound Lipids
(a) Phospholipids, which yield fatty acids, glycerol, amino alcohol sphingosine, phosphoric acid, and nitrogen-containing alcohol upon hydrolysis.
They may be glycerophospholipids or sphingophospholipid depending upon the alcohol group present (glycerol or sphingosine).
(b) Glycolipids, which yield fatty acids, sphingosine or glycerol, and a carbohydrate upon hydrolysis.
They may also be glyceroglycolipids or sphingoglycolipid depending upon the alcohol group present (glycerol or sphingosine).
Derived Lipids
Hydrolysis product of simple and compound lipids is called derived lipids. They include fatty acid, glycerol, sphingosine, and steroid derivatives.
Steroid derivatives are phenanthrene structure that are quite different from lipids made up of fatty acids.
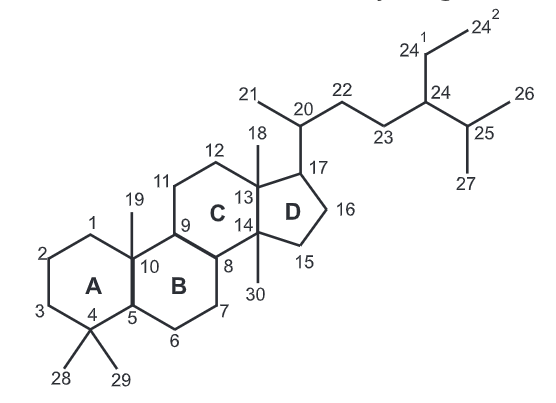
Steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes which alter membrane fluidity; and as_ signaling molecules. Hundreds of steroids are found in plants, animals and fungi. All steroids are manufactured in cells from the sterols lanosterol (eukaryotes) or cycloartenol (plants). Lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene.
The steroid core structure is typically composed of seventeen carbon atoms, bonded in four “fused” rings: three six-member cyclohexane rings (rings A, B and C) and one five-member cyclopentane ring (D ring). Steroids vary by the functional groups attached to this four-ring core and by the oxidation state of the rings. Sterols are forms of steroids with a hydroxy group at position three and a skeleton derived from cholestane. Steroids can also be more radically modified, such as by changes to the ring structure, for example, cutting one of the rings. Cutting ring B produces secosteroids one of which is vitamin D3.
Examples include the lipid cholesterol, the sex hormones estradiol and testosterone and the anti-inflammatory drug dexamethasone.
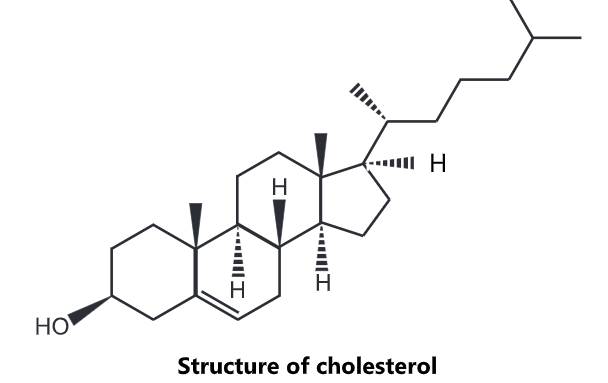
Cholesterol Structure:
Cholesterol is a lipid with a unique structure consisting of four linked hydrocarbon rings forming the bulky steroid structure. There is a hydrocarbon tail linked to one end of the steroid and a hydroxyl
group linked to the other end.
Cholesterol also serves as a precursor for the biosynthesis of steroid hormones, bile acid and vitamin D. Cholesterol is the principal sterol synthesized by all animals. In vertebrates, hepatic cells typically produce the greatest amounts.
Awesome!