Few culture Methods have been discussed.
Types of culture techniques
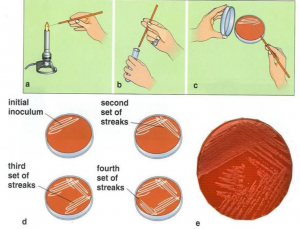
Streak plate method
In streak plate method bacterial suspension is separated on solid media by inoculating needle in various ways.
Methodology
- Sterilize wire loop by holding it in Bunsen burner flame and cool for few second.
- Aseptically remove a loopful of culture with the wire loop.
- Raise the lid of sterile nutrient agar containing plate just high enough to insert the wire loop.
- With free arm movement spread the culture at one corner of plate. Then draw the first streak in first quadrant. Likewise streak in draw 2nd, 3rd, 4th quadrants.
- After each streak sterilize and cool the wire loop.
- There will be more microbe numbers in the first strip. In order to give independent colonies, the last strip could thin out the culture.
- It is then permitted to incubate such an inoculated nutrient agar-petri plate at the appropriate temperature and time. Well-isolated colonies will appear in the final strip. Each colony reflects an organism’s pure growth.
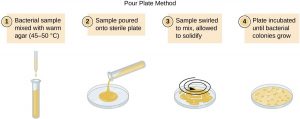
Pour plate method
This involves the following steps
(A)Dilution of sample:
- The dilution of the sample is important to step for this technique.
- Take 1gm/ml of sample, it is mixed with 99ml sterile saline, aseptically. This leads to 1:102 dilution of the sample.
- Using a sterile pipette , add 1ml of this mixture into a tube holding 9ml of sterile saline. It ends in 1:103 dilutions.
- Make several dilutions as above (1:104 to 1:106 ). Select any dilution for further experiments.
(B)Methodology of pour plate:
- Take 1ml mixture from any dilution (e.g. 1:105 ) with the help of sterile pipette.
- Inoculate it at 45 ° C in sterile nutrient agar butt (nutrient agar butt-containing approx. 20 ml medium nutrient agar in tube, held at 50 ° C inliquefied condition).
- Shake the nutrient agar butt, in order to distribute sample properly.
- Pour the content in sterile empty petri-plate and allow solidifying.
- Incubate the plate at proper temperature for 24 hours.
- Analyse the colonies qualitatively and quantitatively. Calculate the number of colonies on agar surface. The number of microorganisms in the diluted sample is given. Multiply the number of colonies by the dilution factor to determine the overall number of microorganisms in the original sample.
Drawbacks:
- The microorganisms are subjected to hot shock because liquid medium is maintained at 45°C temperature.
- This method is unsuitable for isolating psychrophile bacteria.
- This method is tedious, time consuming and requires skilled hands.
Spread plate method
- The process in which bacterial suspension is dispersed evenly by glass spreader on solid agar is the spread plate technique.
- Put 1ml of the sample into the sterile 99 saline containing flask with the aid of a sterile pipette and then add 1ml of this mixture into 9 ml of the saline containing tube. To accomplish a particular dilution, repeat the procedure many times.
- Using a sterile pipette, place 0.1 ml of any prepared dilution on a sterile agar nutrient tray.
- Sterilize a glass spreader and allow it to cool between two burners by flaming after dipping in alcohol.
- With the assistance of a sterilised spreader, then spread the drop of suspension evenly over the agar soil. At the right temperature, incubate the petri dish for 24 hours and observe it the next day.
- Calculate the number of agar-surface colonies. The number of microorganisms in the diluted sample is given. The number of colonies is multiplied by the dilution factor to determine the overall number of microorganisms in the original sample.

Advantages:
- It is possible to enumerate the number of microorganisms in any given suspension by this method.
- Another advantage of this method is that for any purposes a bacterial lawn – dense bacterial growth is required (example in the microbiological assay). This method is appropriate for this purpose.
- One of the advantages is that only surfaces colonies develop and hence are easy to pick up.
Roll tube method:
This system for anaerobic bacteria cultivation was developed by R.E Hugnate. Most bacteria are destroyed by even temporary air contact. It is not necessary to grow certain microbes in routine atmospheric conditions or by general technique. The roll tube process is a commonly used method of separation of anaerobes.
- Take a test tube containing a few mls of molten agar medium.
- The medium should be reduced chemically to remove dissolved oxygen. This is possible by the incorporation of several chemicals like sodium thioglycolate.
- The tube is tightly closed with butyl rubber bung (to maintain anaerobic condition).
- Through injecting them through the rubber stopper with a sterile syringe, the molten agar is inoculated with the necessary dilution of the bacteria source.
- In ice, the tubes are first placed on their sides and rolled until the agar solidifies on the wall of the tube in a thin crust.
- The isolation of the bacteria contributes to this procedure. The bung is extracted and isolated colonies are selected from agar with a needle or capillary tube after incubation as colonies become clear.
- Whenever a tube is opened, entry of air is prevented by continuously passing a stream of CO2 or N2 into the tube. To ensure anaerobic condition, dye like resasurine must be incorporated into the medium.
References
