Cell Disruption: Physical and Chemical Methods
Introduction
- Microbes are protected from the outside environment by rigid cell wall. The cell wall may be extremely hard and the recovery of the intracellular products requires the breakage of the cell wall.
- A number of cell disintegration methods are available but the choice of the method depends on its suitability for the particular substance.
- Though in some cases by the application of a particular method, a specific product can be recovered from inside the cells with greater yield and purity, most of the times these types of applications are not feasible.
- On the other hand, gross disruption of the cell releases a huge number of products and its downstream processing becomes difficult.
- Sometimes application of a particular process is only feasible in the laboratory scale and therefore, the choice of suitable methods for the industrial application is a matter of investigation.
- In recent times, enzymes are the most important intercellular products of interest. But the disruption methods applied for the release of enzymes must keep them active and properly folded, at the same time release yield must be high.
Any potential method of disruption must ensure that-
1) The product should not be denatured by the process.
2) The product should not be hydrolysed by enzymes present in cell.
3) The product should not be contaminated.
The methods fall into two major categories –
I) Physical method
II) Chemical method
I) Physical Method
1) Liquid shear
Liquid shear force for cell disruption is used mostly for the large scale release of enzymes or intracellular products from the microbial cells. The method is based on the shear force generated by cavitations in the cell slurry due to a large pressure drop.
Construction
- The machine consists of a hollow cylinder made up of stainless steel and a piston with an appropriate system of adjustable valves.
- Cell slurry is loaded inside the cylinder and the pressure inside the cylinder is increased to thousands of psi through a system of hydraulics.
- When the cell slurry is allowed to pass through a small orifice, the cells experience a sudden pressure drop.
- That pressure drop causes cavitation and the shock waves so produced disrupt the cells. The amount of pressure drop experienced by the cells has a direct influence on the disruptive release of the enzymes from the cells.
- Therefore, it can be concluded that higher pressure drop is more effective for cell disruption. Mechanical strength of the cell wall, shape and size of the microorganisms also play an important role in achieving effective disruption.
- The process of cell disruption is exothermic and to prevent heat denaturation of the enzyme, an effective cooling system is always needed. The whole process is operated within 0-4°C.
- To make the process less abrasive, it should be operated in multi-pass mode for a longer time. In an industrial process, proper balance has to be made between the maximum release due to effective breakage of the cells and the percentage of released enzyme to achieve cost-effectiveness.
Application
This method is most widely used in large-scale enzyme purification.
2) Solid shear
This is a suitable method for laboratory and can also be operated in a semi-continuous mode on a small scale in the industry. Frozen samples of microorganisms are passed at very low temperature (-25°C) through a small orifice at very high pressure. The breakage occurs due to the liquid-shear and the presence of ice crystals. The process is especially applicable for temperature labile enzymes. By this method 90 % disruption of Saccharomyces cerevisiae can be obtained.
3) Agitation with abrasives
In this method, cells are disrupted with the help of mechanically resistant beads made up of glass, alumina or titanium compounds. Inside a hollow chamber, cells are agitated with beads by a system of agitator shaft. The shear forces so generated cause cell disintegration. The process is exothermic and an efficient cooling system must be associated to prevent thermal denaturation of the enzyme. The process can be applied at a large scale after suitable investigation.
4) Freezing-Thawing
This is a very mild process. Due to repeated freezing and thawing of the microbial cells, ice crystals generate, create pores in the cell wall and facilitate the release of enzyme. The process is applicable in combination with other methods.
It is as low process therefore often used in combination with other techniques. This method is used for separation of β glucosidase from Saccharomyces cerevisiae.
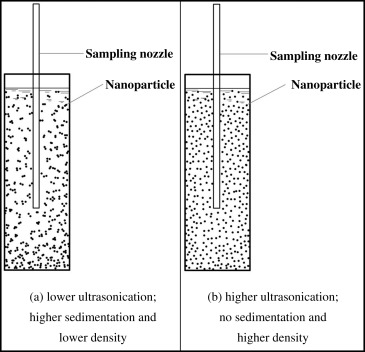
5) Ultrasonication
In this method, high-frequency vibrations are produced at the tip of an ultrasonication probe.These produce waves which cause cell disruption. Ultrasonic waves are used to break the cells at small scale. The process relies on the cavitations generated due to the sonic waves and the shear force generated thereby. A high electrical power is converted into mechanical energy in terms of sonic wave and propagates through a horn in the liquid generating cavitation.
Disadvantages
1) Useful for small scale only.
2) Power requirements are very high.
3) Large heating effect.
4) Probe has short working life.
II) Chemical methods
1) Detergents
- Detergents damage the cell wall by interacting with the lipoproteins of the microbial cell membrane and release the intracellular enzyme.
- The detergents used may be anionic or cationic or Non ionic. The widely used detergents are quaternary ammonium salts, sodium dodecyl sulphate, Triton X-100 etc.
- While applying these substances for the release of enzyme, it has to be kept in mind that the substances can cause protein denaturation and have to be removed from the cell free extract during further purification as soon as possible.
- The use of Triton X-100 is widely known to release membrane bound enzymes. The application of Triton X-100 along with guanidine HCl Is very effective for the release of a number of proteins.
- Unfortunately, the detergents cause some protein denaturation and may need to be removed before further purification stages can be undertaken.
2) Osmotic shock
- Osmotic shock is applied for the mild release for the enzymes from the cells.
- A sudden change in the salt concentration changes the osmotic balance within the cells and the cell is disrupted.
- But the method is not very efficient for the microbial cells having tough cell wall.
- To apply this method for the disruption of microbial cells, generally the cell wall is first made weak by some other method.
- The method has proved to very efficient and unique for the release of luciferase enzyme from Photobacterium fischeri (Osmotic shock caused by sudden change in salt concentration leads to cell disruption.)
3) Alkali treatment
This method is applied for the cell disruption in very limited cases only when the enzyme is highly alkali stable and can tolerate pH up to at least 11.5. Only very few applications of this method are found for the release of the enzyme and one of the classical examples is for the release of L-asparaginase.
