Contents:
Definition
- Enzymes are biocatalysts the catalysts of life. A catalyst is defined as a substance that increases the velocity or rate of a chemical reaction without itself undergoing any change in the overall process.
- Enzymes may be defined as biocatalysts synthesized by living cells. They are protein in nature (exception- RNA acting as ribozyme), colloidal and thermolabile in character, and specific in their action.
Nomenclature and classification
- In the early days, the enzymes were given names by their discoverers in an arbitrary manner. For example, the names pepsin, trypsin, and chymotrypsin convey no information about the function of the enzyme or the nature of the substrate on which they act.
- Sometimes, the suffix-ase was added to the substrate for naming the enzymes e.g. lipase acts on lipids; nuclease on nucleic acids; lactase on lactose.
- These are known as trivial names of the enzymes which, however, fail to give complete information of Enzymes are sometimes considered under two broad categories :
- Intracellular enzymes -They are functional within cells where they are synthesized.
- Extracellular enzymes – These enzymes are active outside the cell; all the digestive enzymes belong to this group.
- The International Union of Biochemistry (IUB) appointed an Enzyme Commission in 1961.
- This committee made a thorough study of the existing enzymes and devised some basic principles for the classification and nomenclature of enzymes.
- Since 1964, the IUB system of enzyme classification has been in force. Enzymes are divided into six major classes in that order).
- Each class on its own represents the general type of reaction brought about by the enzymes of that class.
- Oxidoreductases: Enzymes involved in oxidation-reduction reactions.
- Transferases: Enzymes that catalyze the transfer of functional groups.
- Hydrolases: Enzymes that bring about hydrolysis of various compounds.
- Lyases: Enzymes specialized in the addition or removal of water, ammonia, CO2, etc.
- Isomerases: Enzymes involved in all the isomerization reactions.
- Ligases: Enzymes catalyze the synthetic reactions where two molecules are joined together and ATP is used.
Factor affecting enzymatic action
- The contact between the enzyme and substrate is the most essential prerequisite for enzyme activity.
The important factors that influence the velocity of the enzyme reaction are discussed below:
- Concentration of substrate
- Concentration of enzymes
- Temperature effects
- PH effects
- Activators effects
- Time effects
- Intensive light and radiation
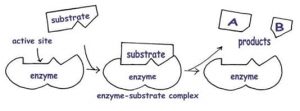
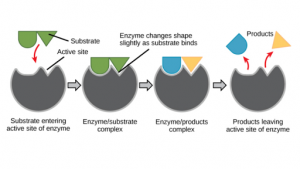
Active site
- Enzymes are big in size compared to substrates which are relatively smaller.
- Evidently, a small portion of the huge enzyme molecule is directly involved in substrate binding and catalysis.
- The active site (or active center) of an enzyme represents the small region at which the substrate(s) binds and participates in the catalysis.
Salient features of the active site
- The existence of an active site is due to the tertiary structure of protein resulting in three-dimensional native conformation.
- The active site is made up of amino acids (known as catalytic residues) which are far from each other in the linear sequence of amino acids (primary structure of the protein).
- For instance, the enzyme lysozyme has 129 amino acids. The active site is formed by the contribution of amino acid residues numbered 35, 52, 62, 63, and 101.
- Active sites are regarded as clefts or crevices or pockets occupying a small region in large enzyme molecules.
Enzyme specificity
- Enzymes are highly specific in their action when compared with chemical catalysts.
- The occurrence of thousands of enzymes in the biological system might be due to the specific nature of enzymes. Three types of enzyme specificity are well-recognized big enzyme molecules.
- Stereospecificity,
- Reaction specificity
- Substrate specificity
Isoenzymes
- The multiple forms of an enzyme catalyzing the same reaction are isoenzymes or isozymes.
- They, however, differ in their physical and chemical properties which include the structure, electrophoretic and immunological properties, Km and Vmax values, pH optimum, relative susceptibility to inhibitors, and degree of denaturation.
