Skip to contentImmunoglobulins: Definition and Types
Introduction
- Immunoglobulins are serum proteins, synthesized by B lymphocytes and plasma cells. During electrophoresis, they migrate in the γ-globulin zone.
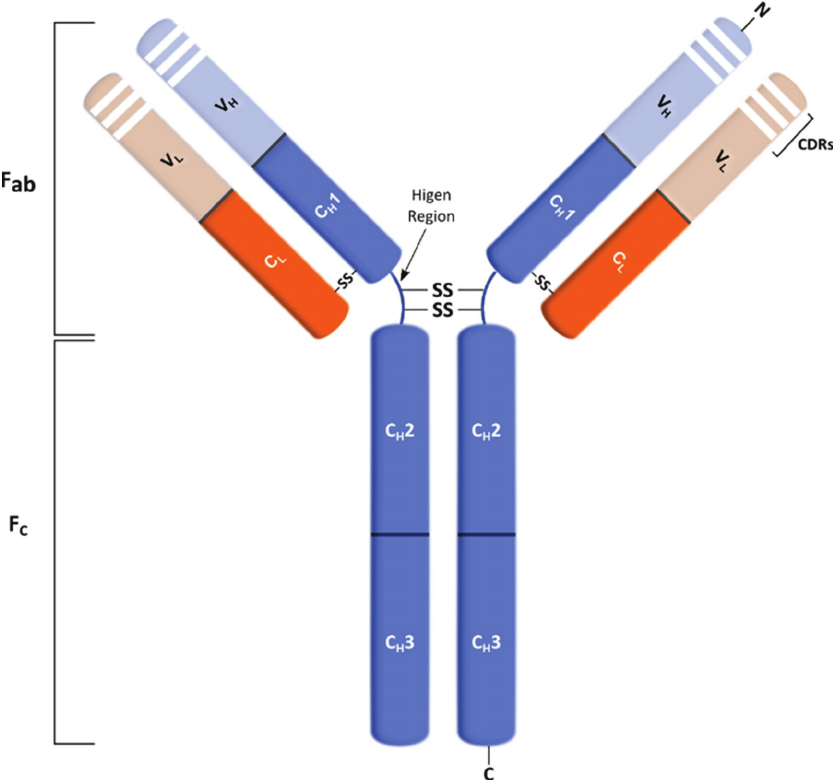
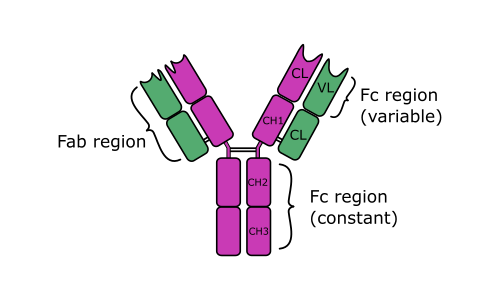
- All immunoglobulin molecules are composed of light and heavy polypeptide chains. The terms “light” and “heavy” originate from their molecular weight – light chains are of molecular mass about 25 kDa, heavy chains – approximately 50 kDa.
- Light (L) chain is of two distinct types, kappa or lambda; the division is based on amino acid differences in their constant regions. Both types occur in all classes of immunoglobulins (IgG, IgM, IgA, IgE, and IgD), but one immunoglobulin molecule contains only one type of L chain.
- Heavy (H) chains are distinct for each of the five immunoglobulin classes. Among them, there are gamma-, mu-, alpha-, delta-, and epsilon types of heavy chains.
- An individual antibody molecule always consists of identical H chains and identical L chains. The simplest antibody molecule of IgG is composed of four polypeptide chains: two H chains and two L chains. These four chains are covalently linked by disulfide bonds.
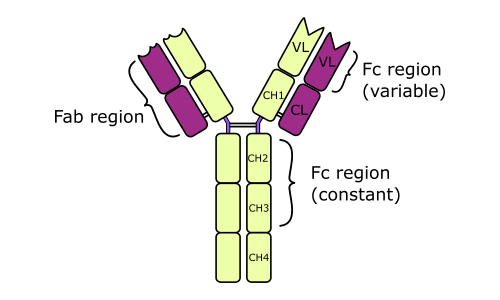
- After treating of immunoglobulin molecule with a proteolytic enzyme (e.g., papain), peptide bonds in the flexible central part of the molecule (its hinge region) are broken. Two identical Fab fragments are formed, which carry the antigen-binding sites, and one Fc fragment, which is involved in the placental transfer, complement fixation, attachment to various cells, and other biologic activities.
- L and H chains are composed of variable regions and constant regions. All the regions contain globular domains.
- An L chain consists of one variable domain (VL) and one constant domain (CL). Most H chains consist of one variable domain (VH) and three or more constant domains (CH). Variable regions are responsible for antigen binding; constant regions are responsible for other functions of Ig.
- In variable regions of both L and H chains, there are three utmost variables (hypervariable) amino acid sequences that form the antigen-binding site. They are known also as complementarity-determining regions (CDRs). Antigen binding is non-covalent, involving van der Waals and electrostatic forces, hydrogen bonds, etc.
- The binding power of the single active site (paratope) of Ab molecule to the corresponding antigenic epitope is termed affinity.
- The binding strength of the whole multivalent antibody molecule to the antigen is known as avidity. It is multiplied depending on antibody valency.
Types of Immunoglobulin
Immunoglobulin G
- Each IgG molecule consists of two L chains and two H chains linked by disulfide bonds. The molecular weight of IgG is about 150 kDa. Its serum concentration is between 8-12 g/l (the mid-level is 10 g/l).
- Because of two identical antigen-binding sites, IgG is divalent. There are four subclasses (IgG1 to IgG4), based on antigenic differences in the H chains and on the number and location of disulfide bonds. The subclass of IgG1 poses about 65% of the total IgG demonstrating the highest defensive potential. IgG2 is directed against polysaccharide antigens and may be an important part of host defense against encapsulated bacteria.
- IgG is the predominant antibody of secondary immune response that ensures the protection against bacteria and viruses. It possesses the highest affinity. IgG activates the complement system via the classical pathway. It also plays a role as an efficient opsonin, activating phagocytosis.
- IgG is the only immunoglobulin, which passes the placenta, thus IgG antibodies prevail in the self-protection of newborns and infants.

Immunoglobulin M
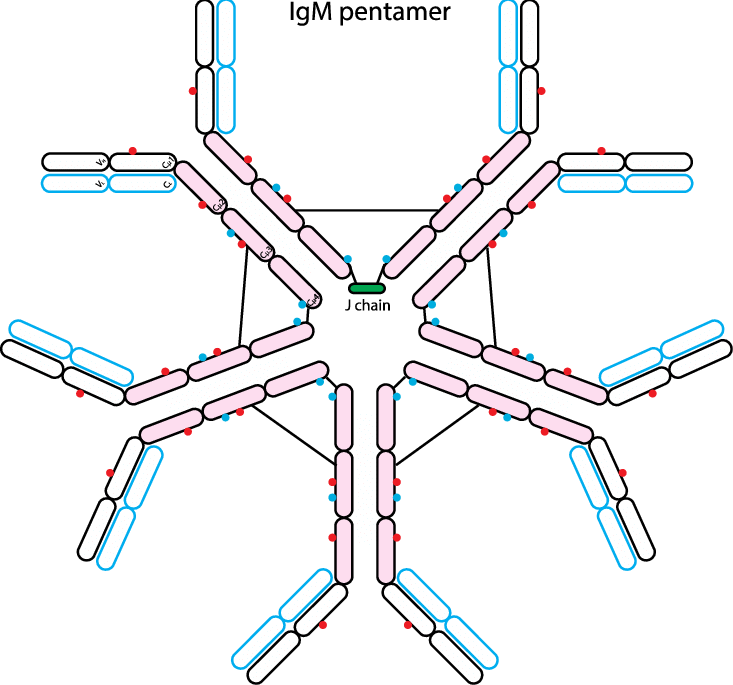
- IgM is the main immunoglobulin produced early in the primary immune response. IgM is present on the surface of the majority of uncommitted В cells. It is composed of five H, L units (each similar to one IgG unit) and one molecule of J (joining) chain.
- The final pentameric molecule (MW 900 kDa) has ten identical antigen-binding sites and thus a valency of 10. Hence, it has the highest avidity of all classes of immunoglobulins. The serum concentration of IgM is from 0.8 to 1.5 g/l.
- It is the most active immunoglobulin in agglutination, complement fixation, and other antigen-antibody reactions.
- It creates the primary line of defense against bacteria and viruses. IgM stimulates phagocytosis (opsonin action) and activates the complement system via the classical pathway.

Immunoglobulin A
- IgA is the major immunoglobulin of the body secretions such as milk, saliva, and tears, secretions of the respiratory, intestinal, and genital tracts. It protects mucous membranes from attacks by bacteria and viruses.
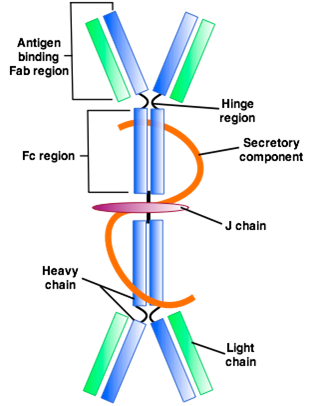
- Each secretory IgA molecule (MW ~ 350 kDa) consists of two H, L units and one molecule each of J chain and secretory component.
- The secretory component binds to IgA dimers and supports their transport across the mucosal epithelial cells. Some IgAs exist in serum as monomeric H-L molecules (with MW of 170 kDa). The serum concentration of IgA is about 1.0-4.0 g/l
- There are at least two subclasses, IgAl and IgA2. Some bacteria (eg, Neisseria) can destroy IgAl by producing a specific IgA-protease. This way they may overcome antibody-mediated resistance of mucosal barriers.

Immunoglobulin E
- The molecular weight of IgE is near 190 kDa. Its serum concentration is extremely low; thus it is expressed in international units (IU). One IU is about 1.5 ng. The normal serum range for IgE is between 0-100 IU per 1 ml of serum.
- IgE is the main antibody in allergy (reagins); they do not penetrate the placenta. The Fc portion of IgE binds to a receptor on the surface of mast cells and basophils.
- This bound IgE acts as a receptor for the antigen. The resulting antigen-antibody complex triggers an allergic response of the immediate (anaphylactic) type with the release of allergy mediators.
- Serum IgE is also typically increased during helminth infections.

Immunoglobulin D
- IgD acts as an antigenic receptor, when present on the surface of mature В lymphocytes. It also occurs on the cells of some lymphatic leukemias.
- Its molecular weight is about 160 kDa. In serum, it is present only in trace amounts (0.04 g/l). IgD does not fixate compliments and doesn’t penetrate the placenta.
- Maybe it is responsible at least in part for anti-viral immunity.