Manifestations Caused By Staphylococcal Toxins
Scalded Skin Syndrome
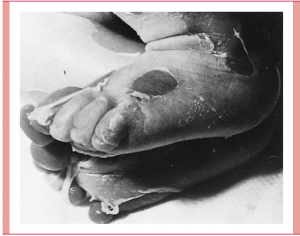
Staphylococcal scalded skin syndrome results from the production of exfoliatin that can be quite minor (e.g., conjunctivitis) in a staphylococcal lesion. In remote locations, erythema and intraepidermal desquamation occurs from which it is not possible to isolate S. aureus . The disease is most common in newborns and children under the age of 5 years. The face, axilla, and groyne tend to be first affected, but all parts of the body may be affected by erythema, bullous formation, and subsequent epithelial sheet desquamation. In adults , especially those who are immunocompromised, the disease occurs occasionally. Staphylococcal scarlet fever, in which erythema occurs without desquamation, and bullous impetigo, in which local desquamation occurs, are milder variants of what is probably the same disease.
Toxic Shock Syndrome
In children, toxic shock syndrome (TSS) was first described but came to public attention during the early 1980s, when hundreds of cases of intravaginal tampon use were reported in young women. High fever, vomiting , diarrhoea, sore throat, and muscle pain characterise the illness. It may progress to severe shock within 48 hours, with evidence of renal and hepatic damage. A skin rash, followed at a deeper level by desquamation than in scalded skin syndrome, may develop. Blood cultures are typically adverse. The outbreak receded with the withdrawal of highly absorbent tampons from certain brands.
Staphylococcal Food Poisoning
Ingesting contaminated food with staphylococcal enterotoxin results in acute vomiting and diarrhoea within 1 to 5 hours. Prostration occurs, but there is usually no fever. Recovery is rapid, except in the elderly and in those with another disease.
DIAGNOSIS
Laboratory procedures to help diagnose staphylococcal infections are quite straightforward. Numerous polymorphonuclear leukocytes and large numbers of Gram-positive cocci in clusters are found in most acute, untreated lesions. Staphylococci grow on aerobically incubated blood agar overnight. For identification, catalase and coagulase tests conducted straight from the colonies are sufficient. Due to the emerging resistance of S. aureus, tests for antibiotic susceptibility are indicated m ultiple antimicrobial , especially methicillin and vancomycin.
Special diagnostic problems occur in deep staphylococcal infections such as osteomyelitis or deep abscesses when the lesion can not be directly aspirated or surgically sampled. In conditions such as acute staphylococcal arthritis, osteomyelitis and endocarditis, blood cultures are generally positive, but less often in localised infections such as deep abscesses.
TREATMENT
Without antimicrobial treatment, many boils and superficial staphylococcal abscesses resolve spontaneously. For optimal results, those that are more extensive, deeper, or in vital organs require a mixture of surgical drainage and antimicrobials. Against S. aureus, penicillins and cephalosporins are active. The peptidoglycan cell wall varies in its susceptibility to staphylococcal-lactamases inactivation. Although penicillin G is the treatment of choice for susceptible strains, due to resistance, penicillin-resistant penicillins (methicillin, nafcillin, oxacillin) and cephalosporins of the first generation are more commonly used. The alternatives are vancomycin, clindamycin, or erythromycin for strains resistant to these agents or for patients with β-lactam hypersensitivity. When staphylococcus is sensitive to both types of agents, there is synergy between cell wall-active antibiotics and aminoglycosides. Such combinations are often used in severe systemic infections when effective and rapid bactericidal action is needed, particularly in compromised hosts.
ANTIMICROBIAL RESISTANCE
Virtually all strains of Staphylococcus aureus were highly susceptible when penicillin was introduced to the general public following World War II. The selection of pre-existing strains capable of producing penicillinase has since shifted these proportions to the point where 80 % to 90% of the isolates are now resistant to penicillin. Penicillinase is encoded by plasmid genes and acts to make the drug unable to bind to its target by opening the β-lactam ring. Resistance to methicillin is based on changes in the β-lactam target, peptidoglycan transpeptidases (often called penicillin-binding proteins, or PBPs). These Staphylococcus aureus (MRSA) methicillin-resistant strains are also resistant to other penicillin-resistant penicillins, such as oxacillin. The most common mechanism is the acquisition of a new transpeptidase gene that has a reduced affinity for β-lactam antibiotics, but is still capable of performing its enzymatic cross-linking peptidoglycan.
There is great geographic variation in the frequency of MRSA. Most American hospitals report MRSA rates of 5 to 25 percent, but in other countries, outbreaks are increasing and resistance rates of over 50% have been reported. There are some difficulties in detecting MRSA; only a small portion of the total population (heteroresistance) may be resistant cells. Tests are usually conducted on methicillin or oxacillin under technical conditions which facilitate the detection of the resistant subpopulation and extrapolate the results to other relevant agents. Oxacillin resistance, for example, is considered to be evidence of resistance to methicillin, nafcillin, dicloxacillin, and to all cephalosporins. Vancomycin is often used to treat MRSA infections that are serious. There is great concern about the recent emergence of Staphylococcus aureus with decreased susceptibility to vancomycin, as these strains are still very rare.
PREVENTION
Preventive measures are aimed at controlling reinfection and, if possible, eliminating carrier status in patients subject to recurrent infection, such as chronic furunculosis. Clothes and bedding that may cause reinfection should be washed to destroy staphylococci (70 ° C or higher) or dry-cleaned at a sufficiently high temperature. In adults, when showering and washing, the use of chlorhexidine or hexachlorophene soaps increases the skin’s bactericidal activity. A combination of nasal creams containing topical antimicrobials (e.g., mupirocin, neomycin, and bacitracin) and oral therapy with antimicrobials concentrated within phagocytes and nasal secretions (e.g., rifampin or ciprofloxacin) may reduce and often eliminate anterior nasal carriage in such individuals or persons found to be the source of an outbreak.
More generally, attempts to reduce nasal carriage among medical staff in an institution are usually fruitless and promote the replacement of susceptible strains with multiresistant strains.
Chemoprophylaxis is effective in surgical procedures, such as replacement of the hip and cardiac valves, where staphylococcal infection can have devastating consequences. During and shortly after surgery, methicillin, cephalosporin, or vancomycin may decrease the chance of intraoperative infection, while minimising the risk of superinfection associated with longer periods of antibiotic administration.
RELATED TOPICS
REFERENCES: