Study Notes on Mineral and Nutrition
- Julius Von Sachs demonstrated that plants can be grown in a defined nutrient solution in complete absence of soil
- This technique is known as hydroponics
- In this method the nutrient elements were added or removed or given in varied concentration.
- After this the mineral solution suitable for the plant growth was obtained.
- This method helped in identification of different elements and their deficiency symptoms.
- This technique is also used for commercial production of vegetables such as tomato, seedless cucumber and lettuce.
ESSENTIAL MINERAL ELEMENTS
- More than 60 mineral elements out of 105 discovered are found in different plants.
- Variety of elements are required for the growth of different kind of plants.
- For instance some plant species accumulate selenium, some others gold, while some plants growing near nuclear test sites take up radioactive Strontium
CRITERIA FOR ESSENTIALITY
- The element must be absolutely necessary for normal growth and reproduction.
- In the absence of the element the plants could not complete their life cycle or set the seeds.
- The element cannot be replaceable by another element.
- This means deficiency of any one element cannot be met by supplying some other element.
- The element must be directly involved in the metabolism of the plant.
- Based on these criteria only a few elements have been found to be absolutely essential for plant growth and metabolism.
- The essential elements are further divided into two broad categories based on their quantitative requirements.
- Macronutrients and Micronutrients.
- The macro nutrients are generally present in plant tissues in large amounts.
- Carbon, hydrogen ,oxygen ,nitrogen, phosphorus, sulphur, potassium, calcium and magnesium are the macronutrients.
- Carbon, hydrogen and oxygen are mainly obtained from CO2 and H2
- The other elements are absorbed from the soil as mineral nutrition.
- Micronutrients are also called as trace elements.
- These are needed in very small amounts.
- Iron , manganese , copper , molybdenum , zinc, boron, chlorine and nickel are the micronutrients.
- There are 17 essential elements in total.
- In addition to these there are some beneficial elements such as sodium silicon cobalt and selenium.
- The beneficial elements are mostly required by the higher plants.
- On the basis of their diverse functions can be grouped into four broad categories.
- Carbon hydrogen oxygen and nitrogen are the elements as components of biomolecules.
- Hence, these are the structural elements of cells.
- The elements that are components of energy related chemical compounds in plants are magnesium in chlorophyll and phosphorus in ATP.
- The essential elements that activate or inhibit enzymes are magnesium zinc and molybdenum.
- Mg2+ is an activator of the critical enzymes in photosynthetic carbon fixation.
- Zn2+ is an activator of alcohol dehydrogenase.
- Molybdenum is the activator of nitrogen is during nitrogen metabolism.
- There are some essential elements available dose can alter the osmotic potential of a cell.
- Potassium plays an important role in the opening and closing of stomata.
ROLE OF MACRO AND MICRONUTRIENTS
- Nitrogen – it is absorbed mainly as NO3– or sometimes also taken up as NO2– or NH4– .
- Nitrogen is particularly required by meristematic tissues and metabolically active cells.
- It is one of the major constituents of proteins , nucleic acids, vitamins and hormones.
- Phosphorus – absorbed by plants from soil in the form of phosphate ions.
- Required for all phosphorylation reactions.
- It is a constituent of cell membranes , certain proteins ,all nucleic acids and
- Potassium – absorbed as potassium ion K+.
- Required in large quantities in the meristematic tissues, buds, leaves and root tips.
- Potassium helps –
- To maintain an anion cation balance in cells
- Involved in protein synthesis
- Opening and closing of stomata
- Activation of enzymes
- Maintenance of the turgidity of cells
- Calcium – absorbed from the soil in the form of calcium ions.
- Required by the meristematic and differentiating tissues.
- Used in the synthesis of cell wall during cell division.
- Present as calcium pectate in middle lamella of the cell wall.
- Also involved in the formation of mitotic spindle.
- Also required for activation of certain enzymes.
- Magnesium – absorbed by plants in the form of divalent magnesium.
- It is a constituent of the ring structure of chlorophyll.
- Magnesium helps :
- Activation of the enzymes for respiration and photosynthesis
- In the synthesis of DNA and RNA.
- To maintain the ribosome structure.
- Sulphur – absorbed in the form of sulphate.
- Main constituent of several coenzymes vitamins and ferredoxin.
- Present in two amino acids , cysteine and methionine.
- Iron – obtained in the form of ferric ions.
- It is a micronutrient and required in larger amounts.
- Activates catalase enzyme.
- Essential for the formation of chlorophyll.
- It is an important constituent of proteins.
- Involved in transfer of electrons like ferredoxin and cytochromes.
- Manganese – absorb in the form of manganous ions.
- Manganese helps :
- In activation of many enzymes involved in photosynthesis respiration and nitrogen metabolism.
- In the splitting of water to liberate oxygen during photosynthesis.
- Zinc – obtained as zinc ions.
- Activates the enzyme carboxylases.
- Needed in the synthesis of auxin.
- Copper – absorbed as cupric ions.
- Associated with certain enzymes involved in redox reactions.
- Essential for the overall metabolism in plants.
- Boron – required for uptake and utilisation of calcium ions.
- Required for membrane functioning pollen germination cell elongation , cell differentiation and carbohydrate translocation.
- Chlorine – absorbed in the form of chloride anion.
- Helps in determining the solute concentration.
- Essential for water splitting reaction in photosynthesis.
- This leads to oxygen evolution.
DEFICIENCY SYMPTOMS OF ESSENTIAL ELEMENTS.
- The concentration of essential elements below which plant growth is retarded is termed as deficiency of the essential elements.
- The element is present below the critical concentration.
- In the absence of any particular element plants show certain morphological changes.
- The changes are the symptoms of the deficiency of any certain element.
- The symptoms vary from element to element.
- After the deficient mineral nutrient is provided to the plant the symptom disappears.
- But it may lead to the death of the plant if the deficiency continues.
- The deficiency symptoms tend to appear first in the older tissues when the element is mobilized.
- When the elements are relatively immobile the deficiency symptoms tend to appear first in the young tissues.
- The deficiency symptoms could be :
- Chlorosis
- Necrosis
- Stunted plant growth
- Premature fall of leaves and buds.
- Inhibition of cell division.
- Chlorosis is the loss of chlorophyll leading to yellowing in leaves.
- Deficiency nitrogen, potassium, magnesium, sulphur, iron, manganese, zinc and molybdenum causes chlorosis.
- Necrosis is death of tissue.
- It is due to deficiency of calcium, magnesium, copper and potassium.
- Lower level of nitrogen, potassium, sulphur, molybdenum causes an inhibition of cell division.
- Deficiency of any element can cause multiple symptoms.
- Same symptoms may be caused by deficiency of more than one elements.
- Different plants respond differently to the deficiency of the same element.
TOXICITY OF MICRONUTRIENTS.
- Micronutrients are always required in lower amounts.
- A moderate increase in micronutrients causes toxicity.
- Micronutrients have a narrow range of concentration at which they are optimum.
- The concentration at which the dry weight of the tissues are reduced by about 10% is considered as toxic.
- The toxic concentration vary widely among different micronutrients.
- The symptoms for the toxicity is difficult to identify.
- Toxicity level of any element also vary for different plants.
- Toxicity may result in the inhibition of the uptake of another element.
- For example manganese inhibit calcium translocation in shoot apex.
MECHANISM OF ABSORPTION OF ELEMENTS.
- The process of absorption can be studied in two main phases.
- In the first phase and initial rapid uptake of Ions into the free space of cells i.e. apoplast takes place.
- This is a passive uptake.
- This movement usually occurs through Ion channels.
- In the second phase the ions are taken in slowly into the inner space which is the simplast of the cells.
- This requires expenditure of metabolic energy so this is an active process .
- The inward movement of Ions into the cells is called influx.
- The outer movement is called efflux.
TRANSLOCATION OF SOLUTES
- Mineral salts are translocated through xylem.
- This takes place along with the ascending stream of water.
- The water stream is pulled up through the plant by transpirational pull.
SOIL AS RESERVOIR OF ESSENTIAL ELEMENTS
- Majority of essential nutrients are available to the roots due to weathering and breakdown of rocks.
- Due to this the soil is enriched with dissolved ions and inorganic salts.
- Soil also harbours nitrogen fixing bacteria.
- Soil also acts like a matrix that stabilized the plant.
METABOLISM OF NITROGEN
- Nitrogen cycle :
- The process of conversion of nitrogen into ammonia is termed as nitrogen cycle.
- Lighting and ultraviolet radiation provide enough energy to convert nitrogen into nitrogen oxides.
- Decomposition of organic nitrogen of dead plants and animals into ammonia is called ammonification.
- Some of these ammonia renters the atmosphere but most of it is converted into nitrate by soil bacteria.
- 2NH3 + 3O2 – 2NO2– + 2H+ + 2H20
2NO2– + O2 – 2NO3–
- Ammonia is first oxidised to nitrate by the bacteria Nitrococcus.
- Nitrite is an oxidised to nitrate with the help of bacteria Nitrobacter.
BIOLOGICAL NITROGEN FIXATION
- Certain prokaryotic are capable of nitrogen fixation.
- Nitrogen fixing microbes could be free living or symbiotic.
- Free living nitrogen fixing aerobic microbes are Azotobacter.
- Azospirillum forms associative relationship with
- Conversion to ammonia from nitrogen molecule in presence of nitrogenase.
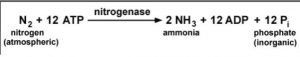
- The energy required for nitrogen fixation by the symbiotic microbes is obtained from the respiration of the host cells.
- Microbes require 16 molecules of ATP to reduce one molecule of nitrogen.
- Rhizobium colonies the host leguminous plant’s root system.
- This causes the roots to form
- The bacteria then begin to fix nitrogen required for the plant.
