Penicillin | The First Antibiotic
- The accidental discovery of penicillin was done by Alexander Flemming in the year 1939 which was later cultured by Florey and Chain in the year 1945.
- It was obtained from Penicillium notatum (now called Penicillium chrysogenum )
- It is the first antibiotic to be used clinically.

Structure
Penicillium consists of two rings.
- Thiozolidone Ring which is sulphur containing with carboxyl group (RING A)
- Beta Lactum Ring (RING B)
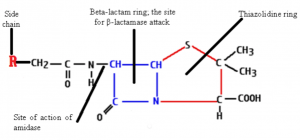
The side chains are attached through the amide linkages.
Mechanism of Penicillin
- The bacterial cell wall is made up of peptidoglycan that protects the cell integrity, shape and prevents macromolecules from entering into the cell.
- It has cross-linking chains of NAG(N-acetylglucosamine) and NAM (N- acetylmuramic acid) components covalently attached through small peptides
- Peptidoglycan is continuously synthesized and remodelled during growth and division.
- Therefore bacteria assemble them into a single macromolecule by synthesizing the peptidoglycan components.
- The peptide cross-linkages are formed by the activity of specific enzymes called transpeptidases or Penicillin-Binding Proteins (PBPs).
- Penicillin contains a four-membered beta-lactam ring inhibits the transpeptidase.
- By mimicking the last two D-alanine residues of the peptide, penicillin is able to bind irreversibly the active site of the transpeptidase, preventing the enzyme from cross-linking the peptidoglycan strands.
- This leads to cell lysis as there won’t be any formation of peptidoglycan and the cell is no more susceptible to osmotic stress.
Penicillin G
- Penicillin G is a broad-spectrum beta-lactam naturally occurring penicillin which is also known as benzylpenicillin
- Benzylpenicillin is more active against Gram-positive bacteria in particular cocci, such as staphylococci, pneumococci, and other streptococci, and bacilli, including Bacillus anthracis, Clostridium perfringens, and Corynebacterium diphtheriae, but less effective against Gram-negative bacteria.
- The penicillin is less effective against Gram-negative bacteria as it has an additional outer membrane, which acts as a selective barrier against penicillin.
- Also, some Gram-negative bacteria have acquired specific genes which encode for penicillinases (beta-lactamases) which inactivate penicillin by hydrolysis of the beta-lactam ring
Second Generation Penicillin
- The Gram-positive strains later emerged a new strain producing penicillinases.
- This lead to the discovery of second-generation penicillin called semi-synthetic beta-lactamase resistant penicillin.
- Ex- Oxacillins, Methicillin, Dicloxacillin
Third Generation penicillin
- The third generation penicillin is more effective against a wider group of Gram-negative bacteria including Haemophilus influenzae, Escherichia coli, Salmonella spp., and Shigella spp
- This included broad-spectrum penicillins known as aminopenicillins
- Ex- Amoxicillin and ampicillin
- These have higher stability towards penicillinases.
- This also includes carboxypenicillins and ureidopenicillins which is effective against Pseudomonas aeruginosa.
Administration
- Penicillin G can be taken either intravenously or intramuscularly.
- Penicillin G administration ensures a continuous low dose of penicillin over 2 to 4 weeks.
- Penicillin G degrades more easily by stomach acid and has a bioavailability of less than 30%.
Adverse Effect
- Penicillin G has adverse effects that include nausea, vomiting, diarrhea, rash, abdominal pain, urticaria, muscle spasms, fever, chills, muscle pain, headache, tachycardia, flushing, tachypnea, and hypotension
Toxicity
- Penicillin has a small risk of toxicity compared to other biologically-active substances
- The doses involve 5g/kg body weight intravenously to cause convulsions in a patient.
References
