Introduction
- Proteins are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues.
- Proteins perform a vast array of functions within organisms, including catalyzing various metabolic reactions, DNA replication, responding to stimuli, providing structural strength to cells and organisms, and transporting molecules from one part to another.
- Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.
- A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide.
- Short polypeptides, containing less than 20-30 residues, are commonly called peptides, or sometimes oligopeptides.
- The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues.
- The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids. Proteins can also work together to achieve a particular function.
- Once formed, proteins only exist for a certain period and are then degraded and recycled by the cell’s machinery.
- A protein’s lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average life span of 1-2 days in mammalian cells.
- Proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism.
- Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle.
- Most proteins consist of linear polymers built from a series of up to 20 different L-α-amino acids. All proteinogenic amino acids possess common structural features, including an a-carbon to which an amino group, a carboxyl group, and a variable side chain are bonded.
- Only proline differs from this basic structure as it contains an unusual ring to the N-end amine group, which forces the CO-NH amide moiety into a fixed conformation.
- The proteins are classified as structural and functional proteins.
Structural Protein
- The largest class is that of structural ones when measured in terms of their total mass. Structural proteins are fibrous proteins.
- The most familiar of the fibrous proteins are the keratins, which form the protective covering of all land vertebrates: skin, fur, hair, wool, claws, nails, hooves, horns, scales, beaks, and feathers.
- Equally widespread, if less visible, are the actin and myosin proteins of muscle tissue. Another group of fibrous structural proteins is silks and insect fibers.
- In addition, there are the collagens of tendons and hides, which form connective ligaments within the body and give extra support to the skin where needed.
Functional Protein
- The meaning of a functional protein is that it has the ability to carry out metabolic processes like breakdown (anabolic) and build up (catabolic) tissues for structural integrity, and also help build the cell walls (peptidoglycan) of plants and some bacteria, as well as humans (peripheral and integral proteins) which help to take in and throw out nutrients from the cell.
- The tertiary structure of a protein is the functional protein.
- Amino acids are molecules used to build proteins. All amino acids have a central carbon atom surrounded by a hydrogen atom, a carboxyl group (COOH), an amino group (NH2), and an R-group. The R-group or side chain differs between the 20 amino acids.
- Amino acids are organic compounds that contain amine (-NH2) and carboxyl (-COOH) functional groups, along with aside chain (R group) specific to each amino acid. The key elements of an amino acid are carbon (C), hydrogen (H), oxygen (O), and nitrogen (N), although other elements are found in the side chains of certain amino acids.
- Twenty of the proteinogenic amino acids are encoded directly by triplet codons in the genetic code and are known as “standard” amino acids.
- The other two (“non-standard” or “non-canonical”) are selenocysteine (present in many prokaryotes as well as most eukaryotes, but not coded directly by DNA), and pyrrolysine (found only in some archaea and one bacterium).
- Nine proteinogenic amino acids are called “essential” for humans because they cannot be produced from other compounds by the human body and so must be taken in as food. Others may be conditionally essential for certain ages or medical conditions. Essential amino acids may also differ between species.
Peptide Bond:
A peptide bond is a chemical bond formed between two molecules when the carboxyl group of one molecule reacts with the amino group of the other molecule, releasing a molecule of water(H20). This is a dehydration synthesis reaction (condensation reaction) and usually occurs between amino acids.
The formation of the peptide bond consumes energy, which, in organisms, is derived from ATP. Peptides and proteins are chains of amino acids held together by peptide bonds.
Amino acids have the following structural properties:
- A carbon (the alpha carbon)
- A hydrogen atom (H)
- Carboxyl group (COOH)
- An Amino group (-NH2)
- A “variable” group or “R” group
Non-polar, polar, and electrically charged are the three properties of side chains used to classify amino acids.
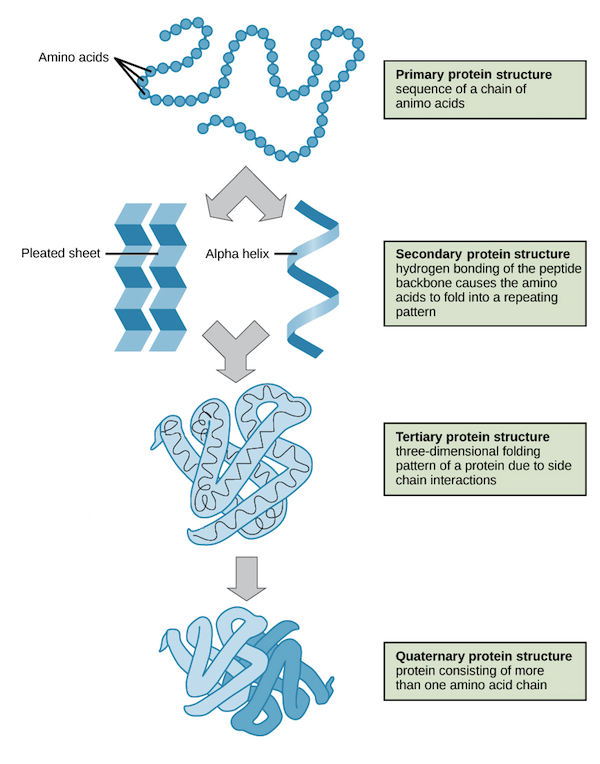
Structural Levels of Proteins
The four levels of protein structure are primary, secondary, tertiary, and quaternary. It is helpful to understand the nature and function of each level of protein structure to fully understand how a protein works.
Primary Structure Protein
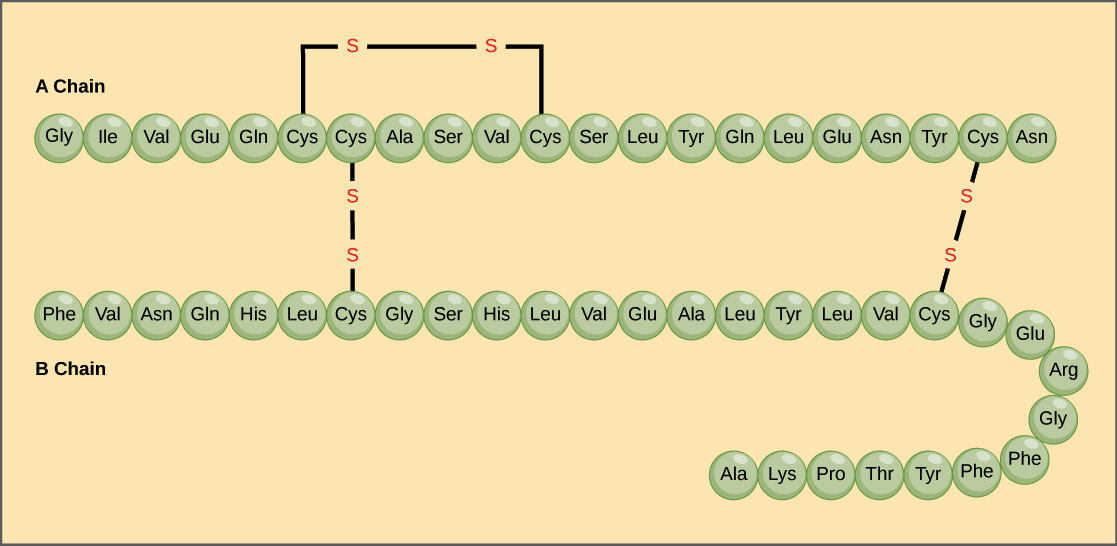
A protein’s primary structure is the unique sequence of amino acids in each polypeptide chain that makes up the protein. Because the final protein structure ultimately depends on this sequence, this is called the primary structure of the polypeptide chain. For example, the pancreatic hormone insulin has two polypeptide chains, A and B.
Secondary Structure Protein
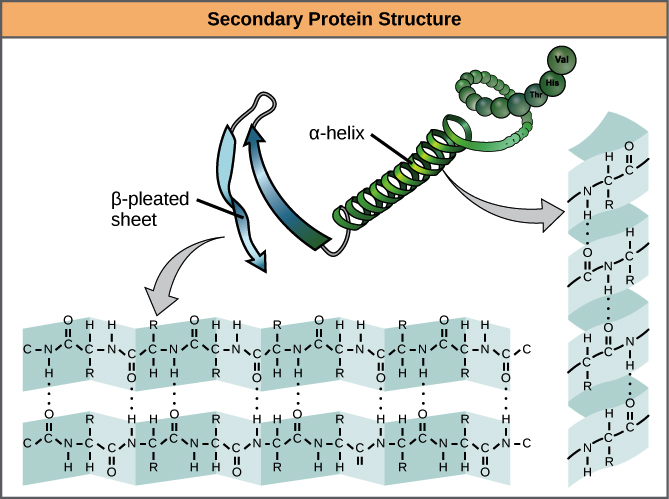
- Secondary structure is local interactions between stretches of a polypeptide chain and includes a-helix and B-pleated sheet structures.
- A protein’s secondary structure arises from interactions between neighboring or nearby amino acids as the polypeptide starts to fold into its functional three-dimensional form.
- Secondary structures arise as H bonds form between local groups of amino acids in a region of the polypeptide chain.
- The most common forms of secondary structure are the α-helix and β-pleated sheet structures and they play an important structural role in most globular and fibrous proteins.
- In the α-helix chain, the hydrogen bond forms between the oxygen atom in the polypeptide backbone carbonyl group in one amino acid and the hydrogen atom in the polypeptide backbone amino group of another amino acid that is four amino acids further along the chain.
- This holds the stretch of amino acids in a right-handed coil. Every helical turn in an alpha helix has 3.6 amino acid residues.
- The R groups (the side chains) of the polypeptide protrude out from the α-helix chain and are not involved in the H bonds that maintain the α-helix structure.
- In β-pleated sheets, stretches of amino acids are held in an almost fully extended conformation that “pleats” or zig-zags due to the non-linear nature of single C-C and C-N covalent bonds. β-pleated sheets never occur alone. They have to be held in place by other β-pleated sheets.
Tertiary Structure Protein
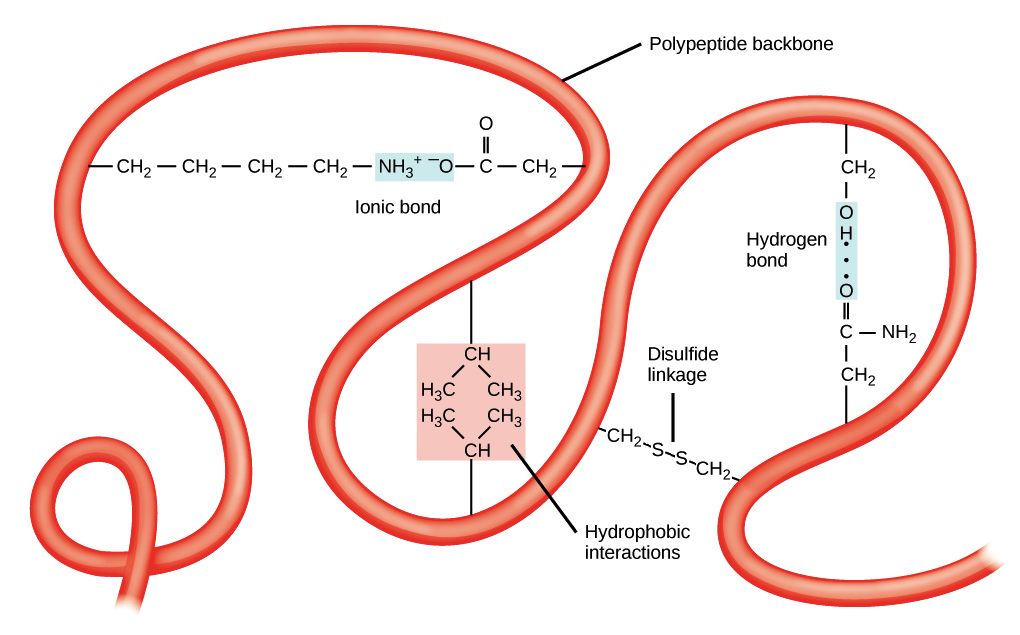
- Tertiary structure is the overall three-dimensional folding driven largely by interactions between R groups.
- The tertiary structure of a polypeptide chain is its overall three-dimensional shape, which occurs once all the secondary structure elements have folded together among each other.
- Interactions between polar, non-polar, acidic, and basic R groups within the polypeptide chain create the complex three-dimensional tertiary structure of a protein.
- When protein folding takes place in the aqueous environment of the body, the hydrophobic R groups of non-polar amino acids mostly lie in the interior of the protein, while the hydrophilic R groups lie mostly on the outside.
- Cysteine side chains form disulfide linkages in the presence of oxygen, the only covalent bond formed during protein folding.
- All of these interactions, weak and strong, determine the final three-dimensional shape of the protein. When a protein loses its three-dimensional shape, it is no longer functional.
Quarternary Structure Protein
- The quarternary structure is the orientation and arrangement of subunits in a multi-subunit protein.
- The quaternary structure of a protein is how its subunits are oriented and arranged with respect to one another.
- As a result, quaternary structure only applies to multi-subunit proteins; that is, proteins made from more than one polypeptide chain. Proteins made from a single polypeptide will not have a quaternary structure.
- In proteins with more than one subunit, weak interactions between the subunits help to stabilize the overall structure. Enzymes often play key roles in bonding subunits to form the final functioning protein.
- For example, insulin is a ball-shaped, globular protein that contains both hydrogen bonds and disulfide bonds that hold its two polypeptide chains together. Silk is a fibrous protein that results from hydrogen bonding between different β-pleated chains.