Acid Fast Staining
Some bacteria are surrounded by a membrane composed of fatty or waxy substances. Therefore, these organisms are not easily stained by simple stains. Once stained they can retain the color even after treatment with strong decolorizing agents like concentrated acid. These organisms are said acid-fast because the stained bacteria are resistant to decolorization with acid alcohol mixture (decolourizing agents).
Acid-fast stain, first introduced by Dr. Paul Ehrlich, also known as the Ziehl-Neelson’s staining. It is named for two German doctors who modified it: the bacteriologist Franz Ziehl (1859-1926) and the pathologist Friedrich Neelson (1854-1898).
Principle:
- The cells of Mycobacteria contain extraordinarily high, about 60% lipid content (mycolic acids and waxes) in their cell wall and are extremely difficult to stain by normal methods like Gram Stain.
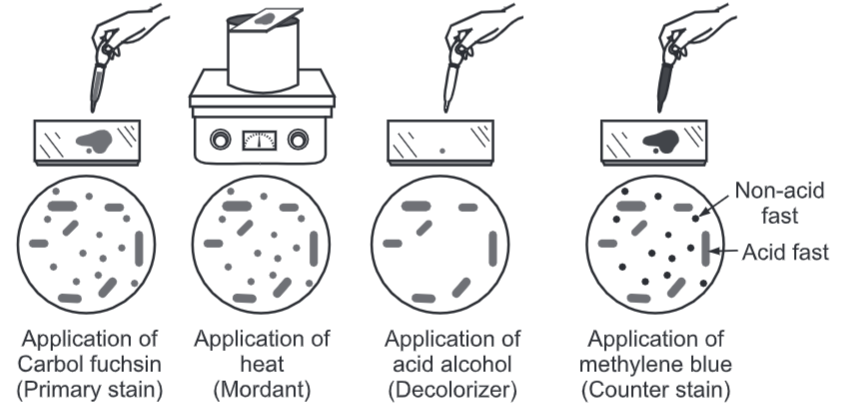
- The phenolic compound Ziehl Neelson’s Carbol Fuchsin (mixture of triphenylmethane dyes, rosaniline, and pararosaniline in aqueous 5% phenol) is used as the primary stain because it is lipid-soluble and penetrates the waxy cell wall.
- Staining by carbol fuchsin is further enhanced by steam heating the preparation to melt the wax and allow the stain to move into the cell.
- Acid alcohol mixture is used to decolorize non-acid-fast cells; acid-fast cells resist this decolorization. The ability of the bacteria to resist decolorization with acid confers acid-fastness to the bacterium.
- Following decolorization, the smear is counterstained with malachite green or methylene blue which stains the background material, providing a contrast color against which the red acid-fast bacilli can be seen.
Procedure:
- Make a thin smear of the material for study and heat fix by passing the slide 3-4 times through the flame of a Bunsen burner or use a slide warmer at 65-75°C. Do not overheat.
- Place the slide on staining rack and pour ZNCF (Ziehl Neelson‘s Carbol fuchsin) over smear and heat gently underside of the slide by passing a flame under the rack until fumes appear (without boiling). Do not overheat and allow it to stand for 5 minutes.
- Rinse smears with water until no colour appears in the effluent.
- Pour decoloriser (20% sulphuric acid + 95% ethanol), wait for one minute and keep on repeating this step until the slide appears light pink in colour (15-20 sec).
- Wash well with clean water.
- Cover the smear with methylene blue or malachite green stain for 1-2 minutes.
- Wash off the stain with clean water.
- Wipe the back of the slide clean and place it in a draining rack for the smear to air-dry (do not blot dry).
- Examine the smear microscopically, using the 100x oil immersion objective.
- Acid-fast staining has importance in examination of sputum samples of patients suffering from tuberculosis. Tissue sections of patients suffering from leprosy can also examined by this method.
Examples of Acid-Fast organisms:
- Mycobacterium spp.: Acid Fast
- Cyst of Cryptosporidium: Acid Fast
- Corynebacterium: Acid fast
- Nocardia spp.: Partial Acid Fast
- Rhodococcus spp.: Partial Acid Fast
- Legionella micdadet: Partially acid fast in tissue