Agrobacterium Mediated Gene Transfer
The most effective vector used in plant transformation is Agrobacterium tumefaciens. It has the benefit of the widespread soil pathogen’s evolved crown gall-inducing DNA transfer mechanism. The natural cause of crown gall disease is Agrobacterium tumefaciens, a rod-shaped, gram-negative bacteria present in the soil. The amino acids, carbohydrates, and organic acids produced by wounded plants attract Agrobacterium tumefaciens. The wounded tissue is binding by a system for polar attachment. The genetic operons that are essential to the commencement of gene transfer expression are triggered during this attachment.
It has been duly established that gene transfer through Agrobacterium is invariably accomplished in the following two methods :
Co-culture with Tissue Explants
- In actual practice, the ‘suitable gene construct’ is strategically inserted carefully very much within the T-region of a disarmed Ti plasmid; and for this one may use either a ‘cointegrate’ or a ‘binary vector’.
- Consequently, the ‘recombinant vector’ is placed carefully in the Agrobacterium that is invariably co-cultured with the plant cells or tissues to be transformed for about 48 hours.
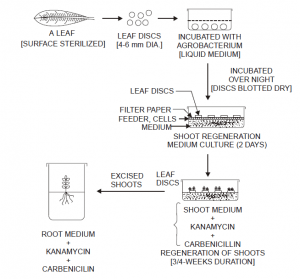
- Importantly, in the instance of several plant species, normally ‘small leaf discs (having a few mm diameter) are cut carefully from the surface of pre-sterilized leaves the subsequently employed for co-cultivation, such as tobacco, tomato, petunia, etc.
- Consequently, the ‘transgene’ thus accomplished essentially comprises of a specific selectable reporter gene e.g., the ‘bacterial neo gene.
- The resulting neo gene is strategically hooked on the appropriate regulatory sequences which are found to be functional in plant cells.
- In actual practice, such a ‘gene’ is invariably termed as a chimeric gene because it predominantly consists of sequences derived from several different genes.
- It has been duly observed that in the course of leaf disc agrobacterium co-culture, a chemical entity is known as acetosyringone duly released by plant cells thereby causing specific induction of the vir genes that particularly affords the plausible transfer of recombinant T-DNA into a plethora of the prevailing plant cells.
- In doing so the resulting T-DNA would eventually get integrated into the plant genome; and hence, the transgene would be expressed. Consequently, the transformed plant cells will become resistant to kanamycin (an antibiotic) on account of the expression of the neo gene.
- After a gap of 48 hours, the treated leaf discs are meticulously transferred onto a generation medium containing suitable concentrations of both kanamycin and carbenicillin as shown in Fig. 1. The two ‘antibodies’ do play specific roles, namely :
(a) Kanamycin — permits exclusively the ‘transformed plant cells’ to undergo division and regeneration of ‘shoots’ in 3-4 weeks duration; and
(b) Carbenicillin — helps in killing Agrobacterium cells only. At this stage, the shoots are adequately separated, rooted, and ultimately transferred into the prepared soil bed.
Salient Features
The prevalent salient features of co-culture with tissue explants areas are enumerated under :
(1) The agrobacterium does infect certain specific monocot plant species and ultimately gives rise to the formation of crown gall cells, such as Asparagus or causes apparent swellings, for instance: Allium cepa, Dioscorea bulbifera, besides Chlorophytum and Narcissus. In most of these instances, the critical production of the opines by the corresponding crown gall cells and swelling tissues was noticed evidently.
(2) Besides, the integration of T-DNA into the genomes of at least two plant species, namely: Dioscorea bulbifera and Oryza Sativa has been well established and amply demonstrated. Nevertheless, the efficiency of transformation is observed to be at a low ebb.
(3) Importantly, an appreciably efficient degree of transformation of the monocot cells may be accomplished by the induction of acetosyringone in the course of co-culture of plant cells with the aid of Agrobacterium.
(4) A few plant species are found to secrete such chemical entities that essentially and precisely inhibit the usual induction of the vir operons by acetosyringone. Example: Secretion of 2, 4-dihydroxy-7-methoxy-1, 4-benzoxazine 3-one (DIMBOA) that specifically inhibits vir gene induction; and this problem may be circumvented by adding an excess of acetosyringone. However, this technique help in the successful transformation in several cereal species e.g., barley, maize, rice, and wheat.
In Planta Transformation
It has been observed that inhibition of Arabidopsis seeds in the freshly prepared cultures of Agrobacterium ultimately gives rise to the stable integration of T-DNA strategically present in the Arabidopsis genome. It seems that the prevailing Agrobacterium cells eventually gain entry into the corresponding seedlings in the course of the germination process, are adequately held up within the plants; and subsequently, when flowers undergo the process of development (i.e., blooming) they ultimately transform either the ensuing ‘zygotes’ or the ‘cells’ which yield zygote.
Another viable and feasible approach is invariably accomplished when the ‘just budding flowers’ of the Arabidopsis plants are duly dipped into a freshly prepared culture of Agrobacterium, and subsequently, a partial vacuum is generated so as to augment and facilitate entry of the corresponding bacterial cells right into the body of the exposed plants. Consequently, the plants are allowed to grow, selfed, and the ‘progeny’ thus obtained are subjected to vigorous methodical screening for the identification of the generated transformants.
Advantages
The various glaring advantages of the two aforesaid approaches of Agrobacterium transformation methodologies are as given below :
(a) absolute elimination of the need for regeneration from the tissue explants,
(b) methodologies involved are relatively not so cumbersome and easy; and, therefore, may be adapted with other plant species with an equally high successful rate,
(c) possess a little risk of ‘somaclonal variation’, although the probability for the mutations independent of stable T-DNA integrations does prevail by virtue of the incidence of the abortive T-DNA insertions.
References
-
https://books.google.com/books?id=sVB5LkRW9N8C -
https://books.google.com/books?id=eDJlDwAAQBAJ -
https://www.mybiosource.com/learn/testing-procedures/agrobacterium-mediated-gene-transfer/#:~:text=Agrobacterium%20tumefaciens%20is%20attracted%20to,start%20of%20gene%20transfer%20expression.