Contents:
Autoimmunity and Autoimmune Diseases
INTRODUCTION
Autoimmunity is a condition in which structural and functional damage is produced by the action of antibodies or immunologically competent cells against the normal components of the body or it is the failure of an organism to recognize its own constituent parts as self, which allows an immune response against its own cells and tissues. For example, Coeliac disease, diabetes mellitus type 1 (IDDM), systemic lupus erythematosus (SLE), Graves disease, etc. Ideally, a minimum of three requirements should be met before a disorder is categorized as truly due to autoimmunity which is as follows:
- Presence of an autoimmune reaction
- Clinical/experimental evidence that such a reaction is not secondary to tissue damage but is of primary pathogenetic significance
- Absence of any other well-defined cause of the disease.
Since autoimmunity implies a loss of self-tolerance, it is important to understand the mechanism of immunological tolerance.
IMMUNOLOGICAL TOLERANCE
Immunological tolerance is a state in which the individual is incapable of developing an immune response to a specific antigen. Self-tolerance refers to the lack of responsiveness to an individual’s antigens. Several mechanisms have been postulated to explain the tolerant state, out of which three are of worthy consideration.
Clonal Deletion
Clonal deletion refers to the loss of self-reactive T and B lymphocytes during their maturation. T cells that bear receptors for self-antigens are deleted within the thymus when these self-antigens are presented to them in relation to self MHC molecules. Therefore, the peripheral T cell pool is deficient in self-reactive T cells. This is the same for B cells when they encounter membrane-bound antigens within the bone marrow.
Clonal Anergy
Clonal anergy refers to prolonged/irreversible functional inactivation of lymphocytes when they encounter antigens under certain conditions. For example, the activation of antigen-specific CD4+ T cells requires two signals:
(i) Recognition of peptide antigen with class I MHC molecules on the surface of antigen-presenting cells (APCs) and
(ii) A set of second co-stimulatory signals provided by the APCs.
This includes binding of T cell-associated molecules (CD28) to its ligand on the APC (called B7). If the antigens presented by cells do not have B7 then the T lymphocyte gets anergic. A special form of peripheral unresponsiveness may occur if a T cell that bears receptors for self-antigens encounters the antigen on a cell that does not express MHC class II molecules.
B cells are also affected by clonal anergy. If B cells encounter antigen before they are fully mature, the antigen receptor complex is endocytosed and such cells can never re-express their immunoglobulin receptors. They are unable to respond to subsequent antigenic stimulation.
Peripheral Suppression by T Cells
Both cellular and humoral factors can actively suppress auto-reactive lymphocytes. Suppressor T cells are CD8+T lymphocytes and secrete cytokines like TGF-beta that downregulate the immune responses.
AUTOIMMUNE DISEASE
An Autoimmune Disease can be defined as a specific and sustained adaptive immune response directed against the self which causes damage to the host. Autoimmune diseases can be divided into two classes depending on the principal clinicopathologic features of each disease. These are systemic autoimmune disorders and organ-specific or localized autoimmune disorders.
Organ-specific Autoimmune Disorder
In an organ-specific autoimmune disorder, the immune response is directed against a target antigen unique to a single organ/gland, so that the manifestations are largely limited to that particular organ only. The cells of the target organs may be damaged directly by cell-mediated or humoral effector responses. Alternatively, the antibodies may over-stimulate or block the normal function of the target organ. Examples are Diabetes mellitus type 1, Grave’s disease, Pernicious anemia, Myasthenia gravis, Hashimoto’s thyroiditis, etc.
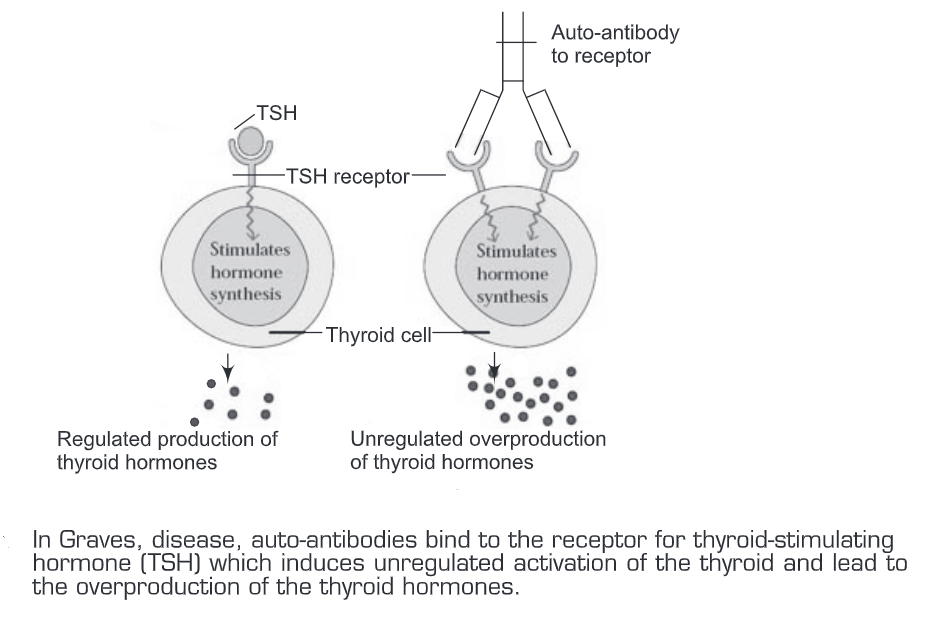
In Grave’s disease, the patient produces auto-antibodies that bind the receptor for TSH and mimic the normal action of TSH, and thus activate adenylate cyclase and results in the production of thyroid hormones. However, the production and binding of auto-antibodies are not regulated and they over-stimulate the thyroid. These auto-antibodies are therefore called long-acting thyroid-stimulating (LATS) antibodies.
Systemic Autoimmune Diseases
In systemic autoimmune diseases, the response is directed toward a broad range of target antigens which involves a number of organs and tissues. These diseases reflect a general defect in immune regulation that results in hyperactive T cells and B cells. Tissue damage by cell-mediated immune responses and from direct cellular damage caused by auto-antibodies or by the accumulation of immune complexes is widespread. Examples are Systemic Lupus Erythematosus (SLE), rheumatoid arthritis, dermatomyositis, etc.
Mechanism of Autoimmune Disease Induction – The induction of autoimmune disease occurs as mentioned below:
- Initially, an infectious agent causes disease.
- We recovered from the disease due to T cell and antibody response.
- A portion of a protein from the infectious agent mimics a self-protein.
- Due to the MHC composition, some T cells specific for infectious agent protein also cross-reacts with self protein.
- T cell becomes “pathogenic”, responds to self-antigen, and recruits other immune cells.
- Finally leads to tissue destruction/damage.
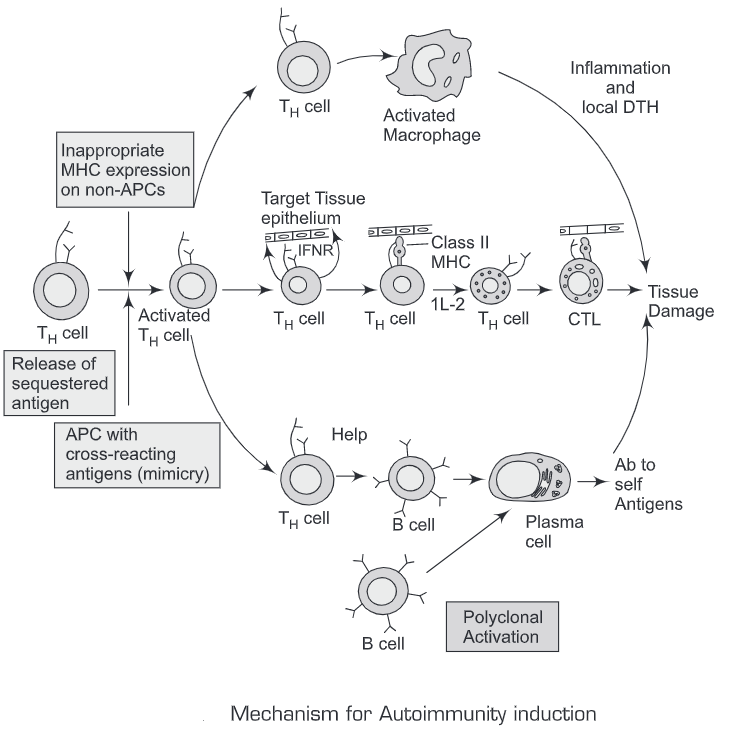
A variety of mechanisms have been proposed for the induction of autoimmune diseases.
The general mechanisms for the T-cell-mediated generation of autoimmune diseases are as follows :
- Molecular mimicry
- Polyclonal B cell activation
- Inappropriate expression of Class IT MHC molecules
- Release of sequestered antigen
- Molecular mimicry— Molecular mimicry means that peptide epitopes of an infectious agent have sequence homology with self-epitopes, therefore the foreign peptides can activate naïve Auto-reactive T cells specific for the corresponding self-epitopes. The presence in a pathogen of a molecule similar to a host antigen could inhibit the immune response of the host against the pathogen because of the immune tolerance towards self-antigens. In molecular mimicry, the infectious agent bears an epitope that is similar to a host antigen, but different enough, so that the host raises an immune response against it. Subsequently, the response could turn against the self-antigen because of cross-reactivity. Therefore molecular mimicry will initiate an autoimmune reaction but this is not by itself enough to cause autoimmune disease. Molecular mimicry is considered an important pathogenetic mechanism in rheumatic fever, type I diabetes mellitus, rheumatoid arthritis, multiple sclerosis, Chagas’ disease, etc.
- Polyclonal B cell activation—Several microorganisms and their products are capable of causing polyclonal activation of B cells. The best investigated among these are bacterial lipopolysaccharide (endotoxin), Gram-negative bacteria, cytomegalovirus, and Epstein-Barr virus (EBV).
- Inappropriate expression of Class II MHC molecules—The pancreatic beta cells of insulin-dependent diabetes mellitus (IDDM) individuals express high levels of class I and class I MHC molecules, whereas healthy beta cells express low levels of class I and do not express class II at all. Similarly, thyroid acinar cells of patients with Graves’ disease express class II MHC molecules on their membranes. This inappropriate expression of class II MHC molecules, which are normally expressed only on antigen-presenting cells, serves to sensitize TH cells to peptides derived from the beta cells or thyroid cells, allowing activation of B cells/TC cells/ sensitization of TH1 cells against self-antigens.
- Release of sequestered antigen— Interaction between the antigen and the immune system is required for tolerance induction. Therefore, any self-antigen that is completely sequestered during embryonic development was not presented to the lymphocytes during their maturation and the subsequent lymphocytes reactive against them were not clonally deleted. Therefore, if these sequestered antigens are later released into circulation they will not be recognized as self and an immune response will develop against these self-antigens.
A variety of other antigen nonspecific mechanisms contribute to this end, and they are collectively known as “bystander activation”. These mechanisms include increased MHC class I or II molecule expression, enhanced processing and presentation of self-antigens, cytokine release with immune activation, direct lymphocyte activation by lymphotropic viruses, and changes in the function of lymphocytes and macrophages. These changes might happen during infections.
Genetic factors in autoimmunity: Evidences are:
(a) Familial clustering of many autoimmune diseases
(b) Linkage of several autoimmune diseases with HLA especially class II antigens.
(c) Induction of autoimmune diseases in transgenic mice.
The exact mechanism by which genes predispose to autoimmunity has not been completely understood, but attention is focused on the relationship of autoimmunity to class I MHC molecules.
At least 2 mechanisms can explain this association:
- CD4+ helper cells are triggered by peptide antigens bound to class II MHC molecules. A class II allele that can bind to a given self-antigen may facilitate an autoimmune response.
- During the process of clonal deletion during embryonic life, if a particular MHC class II molecule presented the antigens poorly to the T cells then the relevant auto-reactive T cell clone will not be deleted. Individuals who inherit such class II molecules may therefore be at an increased risk for developing autoimmunity.
Tests For The Detection Of Autoantibodies
A number of tests can be used for the detection of autoantibodies which are as follows:
- Immunoprecipitation test
- Immunoflourescent tests
- Enzyme-linked immunosorbent assay (ELISA)
- Passive cutaneous anaphylaxis test
- Flocculation and agglutination test
- CFT
Immunoprecipitation Tests: When a soluble antigen combines with its antibody in the presence of electrolytes at a suitable temperature and pH, the antigen-antibody complex forms an insoluble precipitate. When instead of sedimenting, the precipitate remains suspended as floccules, the reaction is known as flocculation. Precipitation can take place in liquid media or in gels such as agar, agarose, or polyacrylamide.
The amount of precipitate that will form will greatly be influenced by the relative proportions of antigens and antibodies. If to the same amount of antiserum in different tubes, increasing amounts of antigens are added, precipitation will be found to occur more rapidly and adequately in one of the middle tubes in which the antigen and antibody are present in optimal or equivalent proportion. In other tubes, precipitation is either weak or absent. For a given antigen-antibody system, the equivalent ratio is constant irrespective of the quantity of the reactants. If the amount of precipitate is plotted in a graph, there are three phases:
- An ascending part (PROZONE or zone of ANTIBODY EXCESS),
- A peak (zone of EQUIVALENCE) and
- A descending part (POSTZONE or zone of antigen excess).
The prozone is of importance in clinical serology as sometimes sera rich in antibody may give false negative precipitation or agglutination result unless seria] dilutions are tested.
Flocculation and agglutination tests: When the antigen is available in a particulate form or if the antigen can be tagged onto particulate materials Viz. erythrocytes, bentonite or latex particles, then on reacting with the antibodies there is clumping of particles within minutes-checked under the microscope
E.g. Haemagelutination Tests- using agglutination of erythrocytes
Bentonite Flocculation Test- using bentonite particles
Latex Agglutination Tests- Using latex particles.
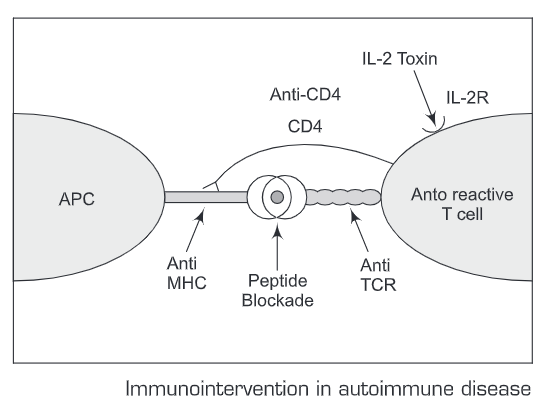
Treatments —Treatment for autoimmune diseases are aimed at reducing only the autoimmune response while leaving the rest of the immune system intact. Some of these approaches are mentioned below:
- Immunosuppressive, anti-inflammatory drugs
- Non-immunological therapies, such as hormone replacement therapy
- T cell vaccination
- Use of monoclonal antibodies
- Peptide blockade of MHC molecules
- Oral antigens
A better understanding of the relationship between infection and autoimmunity may allow the prevention of autoimmune sequelae in some of these conditions.