Introduction
Hybridoma may be defined as — ‘the cell produced by the fusion of an antibody-producing cell and a multiple mycloma cell’.
Importantly, the ‘hybrid cell’ is capable of producing a continuous supply of identical antibodies’.
Another school of thought explains hybridoma as — ‘a hybrid cell obtained by fusing a Blymphocyte with usually a tumour cell of the antibody forming system or of B-lymphocytes (i.e., B-cells), that are invariably known as myclomas.
The hybridoma thus produced essentially possess the inherent capability to produce antibodies by virtue of two cardinal facts, namely : (a) on account of the B-lymphocyte genome ; and (b) due to its capacity for indefinite growth in vitro caused by the tumour (mycloma) cell involved in the fusion. Hence, specific hybridomas are either cultured in vitro or made to pass via the mouse peritoneal cavity to obtain the desired monoclonal antibodies (MABs) ; and this sequential procedures encountered is usually termed as hybridoma technology. It is, however, pertinent to mention here that the development of the hybridoma technology helped to solve insurmountable technical problems intimately associated with the generation of antibodies which are duly recognized to be monospecific in nature because they are monoclonal. In 1984, the Nobel Prize for Physiology and Medicine was bagged by Georges Köhler and Cesar Milstein for their remarkable discovery. The said prize was duly shared with Niels Jerne, who introduced the concept of clonality of the immune response i.e., the theoretical basic foundation upon which the entire methodology is solely based.
The Principle of Monoclonal Antibody Production
The principle of monoclonal antibody production is not only graceful but also stylish. Interestingly, one may ‘capture the particular synthesis’ of a single antibody-forming cell and ‘immortalize’ it in the very tissue culture. Nevertheless, the normal antibody-forming cells cannot be grown and preserved indefinitely (perpetuated) in culture, tumours of the antibody-forming system i.e., myclomas, may be grown indefinitely in culture. Therefore, it is earnestly required to have a method for bringing together in one single cell the two cardinal functionalities, namely : (a) altogether different abilities for the synthesis of a specific antibody ; and (b) ability to grow indefinitely in culture. However, the efforts of Köhler and Milstein succeeded in accomplishing this wonderful aim and objective by allowing to fuse an antibody-forming cell with a mycloma cell, resulting into the formation of a hybrid cell commonly known as a hybridoma.
The crucial problem of selecting the antibody-forming cell of the desired specificity is articulately resolved by fusing relatively huge numbers of the antibody-forming cells on one hand and the mycloma cells on the other. The resulting hybridomas are then meticulously examined (or selected) specifically for those which are exclusively engaged in the synthesis of the ‘antibody’ having the desired (or anticipated) specificity.
Cell Fusion
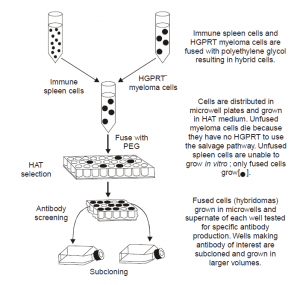
Figure Above↑ vividly illustrates the underlying principle of generating monoclonal antibodies, which are duly accomplished by the fusion of an antibody forming cell (invariably a spleen cell) with a myeloma cell particularly in the presence of one of a variety of fusing agents e.g., polyethylene glycol (PEG).
This ultimately results into the formation of ‘hybridoma’. Separation of fused hybridoma cells from normal spleen cell population :
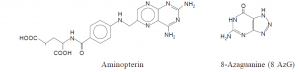
The separation of fused hybridoma cells from the normal spleen cell population is achieved most conveniently by virtue of the glaring fact that the spleen cells normal die off in culture after a short period of time. Interestingly, the unfused myeloma cells and the hybridoma cells are absolutely ‘immortal’; and, therefore, the dire need of an efficacious and specific method is of prime necessity to get rid of the unfused myeloma cells. It is achieved by employing myeloma cells which are killed in the presence of the drug aminopterin.
Just like a plethora of cells, myeloma cells, predominantly make use of two altogether distinct pathways for the nucleotide synthesis, namely : (a) synthetic pathway (major one) ; and (b) salvage pathway (minor one).
Salient Features
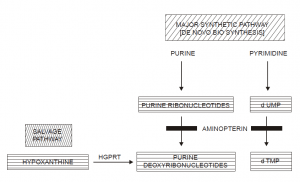
The various salient features of Fig. are as stated below :
(1) Normal cells invariably synthesize nucleotides by employing both pathways.
(2) The very incorporation of the drug 8-azaguanine to the normal cells, enables it to penetrate right into the DNA through a reaction catalyzed by a specific enzyme HGPRT via the salvage pathway. Such cells eventually prove to be fatal as they simply cannot function with an altered base.
(3) Evidently, a variant cell that cannot get along with the salvage pathway by virtue of the fact that it specifically lacks HGPRT should be 8 AzG resistant ; and, therefore, would not be killed by the said drug substance (i.e., 8 AzG).
(4) Therefore, such HGPRT– mutants may be selected particularly with this drug ; and these are the actual myeloma cells generally employed for the desired fusion.
Explanation : In pyrimidine biosynthesis CoFH4 is oxidized to FH2 , thereby using up FH4. In purine biosynthesis CoFH4 is converted to FH4 non enzymatically so that FH4 can be reconstituted to CoFH4. Aminopterin blocks the conversion of FH2 to FH4 so that no more FH4 can be generated. As soon as it has depleted the existing FH4 , the cell can no longer function. As a consequence of the pyrimidine pathway now using up virtually all of the FH4 , the purine pathway also stops ; however, the cell still carries out DNA synthesis via the salvage pathway.
(5) Aminopterin acts on the major synthetic pathway (or De Novo Biosynthesis) by interfering directly with the conversion of dihydrofolate (FH2) to tetrahydrofolate (FH4) and also preventing a series of one-carbon transfers particularly. Hence, in the very presence of aminopterin the cell cannot synthesize nucleotides via the main synthetic patyway ; and, therefore, should take the salvage pathway instead.
(6) A normal cell can still grow in the presence of aminopterin, whereas a HGPRT– cell cannot, perhaps due to the fact that the prevailing HGPRT– mutants cannot carry out the salvage pathway ; and the net effect would be the fatal fate of the ensuing HGPRT– cells in the presence of aminopterin.
(7) In a situation, when HGPRT– myeloma cells get fused with the normal B lymphocytes (or B cells), it has been duly observed that the resulting hybridomas are capable of growing in the presence of aminopterin because the normal cell profusely contributes functional HGPRT.
(8) In another situation, when two chemical entities e.g., hypoxanthine and thymidine, which are recognized as the precursor molecules employed by the enzyme HGPRT in the salvage pathway, are duly incorporated into the medium, evidently the ensuing hybridoma is capable of using the alternate pathway in the synthesis of DNA.
(9) Importantly, the unused normal spleen cells usually die as they are not capable of growing for longer span in the tissue culture, and the unfused myeloma cells get killed by the aminopterin.
(10) As a result the only fused hybridomas are able to grow adequately. This particular phenomena is known as the HAT selection (i.e., hypoxanthine, aminopterin, thymidine selection). Four Important Principles of HAT-Selection :
The four most important principles of HAT selection are as enumerated under :
(a) When the major synthetic pathways are blocked by the folic acid analogue aminopterin, the cell should employ the salvage pathway. This pathway essentially contains the enzyme HGPRT.
(b) HGPRT– myeloma cells may be selected particularly because they can grow in the presence of 8-azaguanine (i.e., 8-AzG). HGPRT+ cells incorporate 8-AzG into DNA. HGPRT– cells do not incorporate the toxic molecule. Hence, HGPRT– cells can grow in its presence.
(c) HGPRT– cells die in the presence of HAT because both the major synthetic pathway and the salvage pathway are blocked virtually.
(d) Fusion of the HGPRT– myeloma cells with the HGPRT+ spleen cells allows growth in HAT by incorporating the missing enzyme for the salvage pathway.
