Contents:
Endospore Staining: Principle, Procedure, Results and Example
Introduction
When vegetative cells of certain bacteria such as Bacillus spp. (aerobic, facultative anaerobic) and Clostridium spp. (anaerobic) are subjected to environmental stresses such as nutrient deprivation, they produce metabolically inactive or dormant form-endospore. The formation of endospore evade the problems associated with environmental stress and helps them to survive.
Endospores are resistant structures produced under unfavourable conditions like heat, cold, chemicals, when carbon and nitrogen become unavailable, drying etc. Sporulation is a defence mechanism to protect the cell when the need arises. Endospores can be formed within different areas of the vegetative cell. They can be central, subterminal, or terminal. Central endospores are located within the middle of the vegetative cell. Terminal endospores are located at the end of the vegetative cell. Sub-terminal endospores are located between the middle and the end of the cell.
The calcium salt of dipicolinic acid (DPA 10 – 15%) is a major constituent of bacterial spores. It is absent in the vegetative cell and is released during spore germination.
Schaeffer-Fulton Method
Principle:
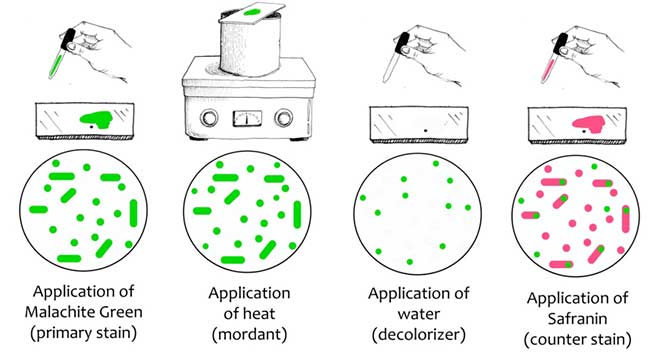
A differential staining technique is used to distinguish between the vegetative cells and the endospores. A primary stain (malachite green) is used to stain the endospores. Because endospores resist staining, the malachite green will be forced into (i.e. malachite green permeate the spore wall) the endospores by heating. In this technique, heating acts as a mordant.
Primary dye malachite green bind relatively weakly to the cell wall and spore wall. After washing with water, dye come out of cell wall however not from spore wall once the dye is locked in. The addition of a counterstain or secondary stain (Safranine) is used to stain the decolorized vegetative cells. Therefore, vegetative cells appear pink and endospores appears green in colour.
Procedure of Endospore Staining:
1. Prepare smears of organisms to be tested for presence of endospores on a clean and grease free glass slide and air dry it.
2. Heat fix the smear.
3. Place a small piece of blotting paper (absorbent paper) over the smear and place the slide (smear side up) on a wire gauze on a ring stand.
4. Heat the slide gently till it starts to evaporate (either by putting the slide on a staining rack that has been placed over a boiling water bath or via Bunsen burner).
Spore staining procedure:
5. Remove heat and reheat the slide as needed to keep the slide steaming for about 3-5 minutes. As the paper begins to dry add a drop or two of malachite green to keep it moist, but don’t add so much at one time that the temperature is appreciably reduced.
6. After 5 minutes carefully remove the slide from the rack using a clothes pin.
7. Remove the blotting paper and allow the slide to cool to room temperature for 2 minutes.
8. Rinse the slide thoroughly with tap water (to wash the malachite green from both sides of the microscope slide).
9. Stain the smear with Safranine for 2 minutes.
10. Rinse both sides of the slide to remove the secondary stain and blot the slide or air dry.
11. Observe the slide under oil immersion lens.
Dorner Method:
Procedure:
1. In this staining process, 2 – 3 ml broth culture of microorganism and equal volume of Basic fuchsin are heated in a water bath at 100°C for 10 minutes. The extensive boiling softens the structure of the bacterial spores and the basic fuchsin gets into the spores.
2. After the boiling process, the microbial culture-basic fuchsin stain is cool for some time which hardens the outer covering of the spore.
3. In order to give a colour to the background and to differentiate between the vegetative cells and endospores, a thin film of loopful of the microbial culture-basic fuchsin stain is prepared and 2 drops of nigrosine are added on a clean glass slide. Since nigrosine is negatively charged, the bacterial cells cannot take up nigrosine.
Result:
After staining the vegetative cells appear become colourless, the endospores stains as red which can be present as terminal or sub-terminal. The background is stained as dark by the Nigrosine stain.
Examples of endospore-forming bacteria:
Bacillus, Actinomyces, Alkalibacillus, Ammoniphilus, Anaerobacter, Sporolactobacillus, Brevibacillus, Clostridium, Coxiella burnetii, Desulfotomaculum, Dendrosporobacter, Desulfosporosinus, Heltobactertum, Thermoactinomyces, Thermoalkalibacillus, Thermoanaerobacter, Thermoanaeromonas, Thermobacillus.