Contents:
- Introduction
- Taxonomy and Classification
- Morphology and Structure
- Cell Wall Composition
- Genome
- Metabolism and Growth
- Pathogenesis
- Virulence Factors
- Transmission and Epidemiology
- Clinical Manifestations
- Diagnosis
- Treatment
- First-Line Drugs and Their Mechanisms
- Prevention and Control
- Drug Resistance
- Research and Development
- Mycobacterium tuberculosis in the Laboratory
- FAQs About Mycobacterium tuberculosis
- References
Introduction
Mycobacterium tuberculosis is the pathogenic bacterial species responsible for tuberculosis (TB), one of history’s most devastating infectious diseases. Despite medical advances, TB remains a significant global health concern, particularly in developing nations.
Taxonomy and Classification
Mycobacterium tuberculosis belongs to:
- Domain: Bacteria
- Phylum: Actinobacteria
- Class: Actinobacteria
- Order: Actinomycetales
- Family: Mycobacteriaceae
- Genus: Mycobacterium
- Species: M. tuberculosis
It is part of the Mycobacterium tuberculosis complex (MTBC), which includes several closely related species that can cause tuberculosis in humans and animals.
Morphology and Structure
M. tuberculosis has several distinctive structural features that contribute to its pathogenicity and survival:
| Structural Feature | Description | Significance |
|---|---|---|
| Cell Shape | Rod-shaped bacillus (0.2-0.6 μm wide, 1-10 μm long) | Classic morphology for identification |
| Cell Wall | Unique waxy composition rich in mycolic acids | Provides impermeability and resistance to many antibiotics, stains, and disinfectants |
| Acid-Fastness | Retains carbol fuchsin dye even after acid wash | Key diagnostic feature used in Ziehl-Neelsen staining |
| Gram Reaction | Weakly Gram-positive but difficult to stain | Traditional Gram staining is not useful for identification |
| Capsule | No true capsule but has outer polysaccharide layer | Contributes to virulence and immune evasion |
| Spores | Non-spore forming | Relies on other survival mechanisms |
| Growth | Slow-growing (generation time 15-20 hours) | Causes diagnostic delays and prolonged treatment time |
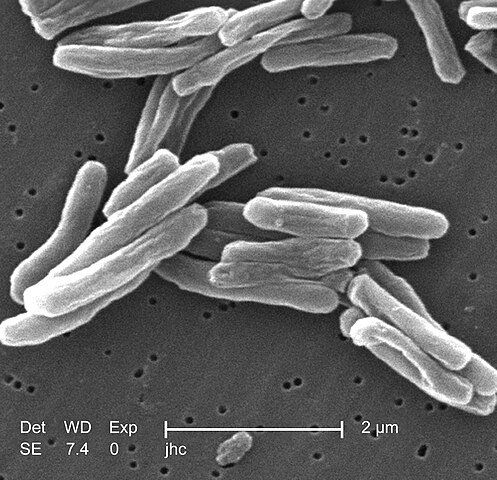
Cell Wall Composition
The cell wall of M. tuberculosis deserves special attention as it is central to the bacterium’s pathogenicity and resistance:
- Peptidoglycan Layer: Forms the basic structural component
- Arabinogalactan Layer: Polysaccharide layer attached to peptidoglycan
- Mycolic Acid Layer: Complex fatty acids (C60-C90) that create a waxy, hydrophobic outer surface
- Surface Lipids: Including cord factor (trehalose dimycolate), wax-D, and others
- Cell Wall Proteins and Glycoproteins: Function in virulence and immune modulation
Genome
- Genome Size: Approximately 4.4 million base pairs
- GC Content: High (65%)
- Genes: Approximately 4,000 coding sequences
- Special Features:
- Contains many genes for lipid metabolism
- Lacks classic virulence factors like toxins
- Has numerous PE/PPE genes (6-10% of genome) thought to be involved in antigenic variation
- Contains insertion sequences that contribute to genomic plasticity
Metabolism and Growth
M. tuberculosis has unique metabolic characteristics that make it challenging to treat:
| Aspect | Characteristics | Clinical Significance |
|---|---|---|
| Respiration | Obligate aerobe | Preferentially infects oxygen-rich tissues like lung apices |
| Growth Rate | Extremely slow (15-20 hour doubling time) | Requires prolonged treatment; difficult to culture |
| Nutritional Requirements | Fastidious | Complex media required for laboratory cultivation |
| Metabolic Flexibility | Can adapt to various nutrient-limited environments | Ability to persist in host tissues during latency |
| Lipid Metabolism | Central to both catabolism and anabolism | Many TB drugs target lipid synthesis pathways |
| Dormancy Capability | Can enter non-replicating persistence state | Basis for latent TB infection |
Pathogenesis
The disease progression of tuberculosis follows a complex pattern:
- Initial Infection:
- Inhalation of aerosol droplets containing M. tuberculosis
- Bacilli reach alveoli in the lungs
- Engulfed by alveolar macrophages but resist killing
- Innate Immune Response:
- Macrophages attempt to destroy bacteria via phagolysosome fusion
- M. tuberculosis inhibits phagosome-lysosome fusion
- Bacteria multiply within macrophages
- Granuloma Formation:
- T-cells and macrophages form a structured lesion around infected cells
- Caseous necrosis develops in the center
- Bacteria become walled off but can remain viable
- Disease Progression:
- In 90-95% of cases: contained infection (latent TB)
- In 5-10% of cases: progressive disease (active TB)
- Reactivation:
- Can occur years after initial infection
- Often triggered by immunosuppression
- Leads to active disease with viable bacteria spreading to new areas
Virulence Factors
M. tuberculosis employs several mechanisms to establish and maintain infection:
| Virulence Factor | Function | Effect on Host |
|---|---|---|
| Cell Wall Lipids | Impermeability and immune modulation | Protection from host defenses |
| Cord Factor | Inhibits neutrophil migration | Impairs innate immunity |
| Protein Kinase G (PknG) | Prevents phagosome-lysosome fusion | Allows intracellular survival |
| Catalase-Peroxidase (KatG) | Detoxifies reactive oxygen species | Protects against oxidative stress |
| ESX Secretion Systems | Secretes virulence proteins | Manipulates host immune response |
| ManLAM | Inhibits phagosome maturation | Promotes intracellular survival |
| Phthiocerol Dimycocerosates (PDIMs) | Mask pathogen-associated molecular patterns | Evades immune recognition |
Transmission and Epidemiology
Understanding the transmission dynamics is crucial for controlling TB:
- Mode of Transmission: Primarily airborne via respiratory droplets
- Infectiousness: Patients with active pulmonary TB are most infectious
- Risk Factors for Transmission:
- Proximity and duration of contact with infectious cases
- Poor ventilation
- Inadequate infection control measures
- Global Burden:
- Approximately 10 million new cases annually
- About 1.4 million deaths each year
- Highest burden in South-East Asia, Africa, and Western Pacific regions
- High-Risk Populations:
- People living with HIV/AIDS
- Malnourished individuals
- Diabetics
- Smokers
- People living in overcrowded conditions
Clinical Manifestations
TB can present with diverse clinical features:
| Type | Manifestations | Notes |
|---|---|---|
| Pulmonary TB | Chronic cough, hemoptysis, chest pain, weight loss, fever, night sweats | Most common form (85% of cases) |
| Extrapulmonary TB | Varies by organ system affected | Occurs in 15-20% of cases, more common in immunocompromised |
| Lymphatic TB | Cervical lymphadenopathy (“scrofula”) | Most common extrapulmonary form |
| Skeletal TB | Back pain, joint destruction, deformity | Often affects spine (Pott’s disease) |
| Genitourinary TB | Dysuria, hematuria, infertility | Can lead to renal failure if untreated |
| Central Nervous System TB | Headache, altered mental status, neurological deficits | Includes TB meningitis and tuberculomas |
| Miliary TB | Multi-organ involvement, severe systemic symptoms | Results from hematogenous dissemination |
| Latent TB | Asymptomatic | No symptoms but positive TB skin test |
Diagnosis
Accurate diagnosis is essential for effective TB control:
- Clinical Assessment:
- Symptom evaluation
- Physical examination
- Chest X-ray
- Medical history
- Microbiological Methods:
- Acid-fast bacilli (AFB) smear microscopy
- Culture on specialized media (Löwenstein-Jensen, MGIT)
- Drug susceptibility testing
- Molecular Diagnostics:
- Nucleic acid amplification tests (NAATs)
- GeneXpert MTB/RIF assay
- Line probe assays for drug resistance
- Immunological Tests:
- Tuberculin skin test (TST/Mantoux)
- Interferon-gamma release assays (IGRAs)
- Histopathological Examination:
- Granulomatous inflammation
- Caseous necrosis
Treatment
TB treatment requires a multi-drug approach to prevent resistance:
| Treatment Phase | Duration | Drugs | Purpose |
|---|---|---|---|
| Intensive Phase | 2 months | Isoniazid, Rifampin, Pyrazinamide, Ethambutol (HRZE) | Rapidly reduce bacterial load |
| Continuation Phase | 4 months | Isoniazid, Rifampin (HR) | Eliminate remaining bacilli |
| MDR-TB Regimen | 9-24 months | Combination of second-line drugs | Address resistance to first-line drugs |
| XDR-TB Regimen | 12-24+ months | Individualized based on susceptibility | Address resistance to multiple drugs |
| Latent TB Treatment | 3-9 months | Isoniazid or other shorter regimens | Prevent progression to active disease |
First-Line Drugs and Their Mechanisms
| Drug | Mechanism of Action | Common Side Effects |
|---|---|---|
| Isoniazid (INH) | Inhibits mycolic acid synthesis | Peripheral neuropathy, hepatotoxicity |
| Rifampin (RIF) | Inhibits RNA polymerase | Orange bodily fluids, hepatotoxicity, drug interactions |
| Pyrazinamide (PZA) | Acidifies cytoplasm, multiple targets | Hepatotoxicity, hyperuricemia, arthralgia |
| Ethambutol (EMB) | Inhibits arabinogalactan synthesis | Optic neuritis, color vision changes |
| Streptomycin | Inhibits protein synthesis (30S ribosomal subunit) | Ototoxicity, nephrotoxicity |
Prevention and Control
Effective TB control requires a comprehensive approach:
- Case Detection and Treatment:
- Active case finding
- Directly Observed Treatment Short-course (DOTS)
- Contact tracing
- Vaccination:
- Bacillus Calmette-Guérin (BCG) vaccine
- Variable efficacy (0-80%)
- Most effective against severe forms in children
- Infection Control Measures:
- Proper ventilation
- Ultraviolet germicidal irradiation
- Personal protective equipment
- Latent TB Management:
- Screening high-risk populations
- Preventive therapy for latent infection
- Public Health Strategies:
- Education and awareness
- Addressing social determinants
- Integration with HIV services
Drug Resistance
Drug-resistant TB poses a major challenge:
| Type | Definition | Treatment Challenge |
|---|---|---|
| Mono-resistant TB | Resistance to one first-line drug | May require regimen adjustment |
| Multidrug-resistant TB (MDR-TB) | Resistance to at least isoniazid and rifampin | Requires longer treatment with more toxic drugs |
| Extensively drug-resistant TB (XDR-TB) | MDR-TB plus resistance to fluoroquinolones and at least one injectable second-line drug | Very difficult to treat, high mortality |
| Totally drug-resistant TB (TDR-TB) | Resistant to all tested first- and second-line drugs | Extremely limited treatment options |
Research and Development
Current areas of TB research include:
- New Drug Development:
- Bedaquiline, delamanid, and pretomanid
- Novel drug targets and mechanisms
- Improved Diagnostics:
- Point-of-care tests
- Biomarkers for disease progression
- Vaccine Development:
- Improved alternatives to BCG
- Therapeutic vaccines
- Host-Directed Therapies:
- Immunomodulatory approaches
- Targeting host factors
Mycobacterium tuberculosis in the Laboratory
Working with M. tuberculosis requires special considerations:
- Biosafety Level: BSL-3 facilities required
- Culture Media: Specialized media like Löwenstein-Jensen, Middlebrook 7H10/7H11
- Identification: Acid-fast staining, biochemical tests, molecular methods
- Decontamination: Chemical methods needed for specimens from non-sterile sites
- Growth Characteristics: Slow-growing (3-8 weeks), dry, rough colonies
FAQs About Mycobacterium tuberculosis
Q1: What makes tuberculosis treatment so long compared to other bacterial infections? A: The lengthy treatment is necessitated by M. tuberculosis’s unique biological properties: its extremely slow growth rate (15-20 hour doubling time versus minutes for most bacteria), its ability to enter a dormant state where it’s less metabolically active and thus less susceptible to antibiotics, and its waxy cell wall that limits drug penetration. This combination of factors means drugs must be administered for months to effectively eliminate all bacteria, including those in different metabolic states.
Q2: Can someone have tuberculosis without showing symptoms? A: Yes, this is called latent tuberculosis infection (LTBI). In this state, individuals are infected with M. tuberculosis but the bacteria are contained within granulomas and not actively replicating. These individuals have no symptoms, cannot transmit the disease, and may never develop active TB. However, they do carry a 5-10% lifetime risk of the latent infection progressing to active disease, with the risk being higher in the first few years after infection and in immunocompromised individuals.
Q3: Why is TB associated with HIV, and what special considerations exist for co-infected patients? A: HIV weakens the immune system, particularly CD4+ T cells that are crucial for containing M. tuberculosis infection. This impairs granuloma formation and maintenance, allowing latent infections to reactivate or new infections to progress rapidly. TB-HIV co-infected patients require special management including: antiretroviral therapy, adjusted TB treatment regimens to manage drug interactions, careful monitoring for immune reconstitution inflammatory syndrome (IRIS), and consideration of drug absorption issues in advanced HIV disease.
Q4: What is the difference between drug-resistant TB and MDR-TB? A: Drug-resistant TB refers to tuberculosis caused by M. tuberculosis strains resistant to any anti-TB drug. MDR-TB (multidrug-resistant TB) specifically refers to tuberculosis resistant to at least the two most powerful first-line drugs: isoniazid and rifampin. MDR-TB requires treatment with second-line drugs that are more toxic, less effective, more expensive, and must be taken for a longer duration (9-24 months versus 6 months for drug-susceptible TB).
Q5: Why is tuberculosis called the “great mimicker” in clinical medicine? A: Tuberculosis has earned this nickname because it can affect virtually any organ system and produce symptoms that mimic many other diseases. Pulmonary TB can resemble pneumonia, lung cancer, or chronic obstructive pulmonary disease. Extrapulmonary TB can present as meningitis, arthritis, pericarditis, lymphadenitis, or a variety of other conditions depending on the site of infection. This diverse clinical presentation often leads to diagnostic delays and underscores the importance of considering TB in differential diagnoses, particularly in high-risk populations.
Q6: What are the “cough etiquette” and infection control measures for TB patients? A: Patients with active pulmonary TB should practice proper cough etiquette by covering their mouth and nose with tissues when coughing or sneezing and discarding used tissues properly. They should wear surgical masks when around others, especially in healthcare settings. Ideally, TB patients should be isolated in negative pressure rooms until they are no longer infectious (usually after approximately two weeks of effective treatment). Visitors and healthcare workers should wear appropriate respiratory protection (N95 respirators or equivalent) when in close contact with infectious TB patients.
Q7: Can M. tuberculosis survive outside the human body? A: Yes, M. tuberculosis can survive outside the human body for extended periods under certain conditions. In airborne droplet nuclei, it can remain viable for several hours in dark, moist environments. On surfaces, it may survive for days to weeks, particularly if protected from direct sunlight (UV light) which damages the bacteria. However, it cannot replicate outside of a host. This environmental persistence contributes to transmission risk but is less significant than direct person-to-person airborne transmission.
Q8: How does the BCG vaccine work, and why isn’t it more widely used in the United States? A: The BCG vaccine contains a live attenuated strain of Mycobacterium bovis that stimulates immunity against M. tuberculosis without causing disease. It primarily generates cell-mediated immunity and is most effective at preventing severe forms of TB in children. The United States does not routinely administer BCG because: 1) the overall risk of TB infection is low, 2) the vaccine has variable efficacy in preventing pulmonary TB in adults, 3) vaccination causes false-positive tuberculin skin test results, complicating the diagnosis of latent TB infection, and 4) targeted testing and treatment of latent TB is considered more cost-effective in low-incidence settings.
References
- World Health Organization. (2023). Global Tuberculosis Report 2023. https://www.who.int/teams/global-tuberculosis-programme/tb-reports
- Centers for Disease Control and Prevention. (2023). Core Curriculum on Tuberculosis: What the Clinician Should Know. https://www.cdc.gov/tb/education/corecurr/index.htm
- Furin J, Cox H, Pai M. (2019). Tuberculosis. The Lancet, 393(10181), 1642-1656. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)30308-3/fulltext
- Pai M, Behr MA, Dowdy D, et al. (2016). Tuberculosis. Nature Reviews Disease Primers, 2, 16076. https://www.nature.com/articles/nrdp201676
- Cole ST, Brosch R, Parkhill J, et al. (1998). Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature, 393(6685), 537-544. https://www.nature.com/articles/31159
- Gagneux S. (2018). Ecology and evolution of Mycobacterium tuberculosis. Nature Reviews Microbiology, 16(4), 202-213. https://www.nature.com/articles/nrmicro.2018.8
- Getahun H, Matteelli A, Chaisson RE, Raviglione M. (2015). Latent Mycobacterium tuberculosis infection. The New England Journal of Medicine, 372(22), 2127-2135. https://www.nejm.org/doi/full/10.1056/NEJMra1405427
- Cambier CJ, Falkow S, Ramakrishnan L. (2014). Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell, 159(7), 1497-1509. https://www.cell.com/cell/fulltext/S0092-8674(14)01507-X
- Dheda K, Gumbo T, Maartens G, et al. (2017). The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. The Lancet Respiratory Medicine, 5(4), 291-360. https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(17)30079-6/fulltext
- Ehrt S, Schnappinger D. (2009). Mycobacterial survival strategies in the phagosome: defence against host stresses. Cellular Microbiology, 11(8), 1170-1178. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1462-5822.2009.01335.x
