Reproduction,Gametogenesis And Fertilization
▶Asexual Reproduction
In asexual reproduction the parent divides, fragments or buds to give rise to a varying number of genetically identical offspring. This ‘natural cloning’ is the result of mitotic cell division. The simplest form of asexual reproduction is seen in fission where the parent divides into two (binary fission) or more parts, each becoming an independent organism. Among the Protoctista, binary fission occurs in Amoeba spp.; in metazoan animals, platyhelminth flatworms exhibit reproduction by fission. Budding is seen in Cnidaria (e.g. Hydra spp.) and Urochordata (e.g. Pyrosoma sp.): buds on the parent’s body differentiate and break free to form new organisms or remain attached to form components in a colony.
Lizards can regenerate new tails if the original is lost, starfishes can regenerate a whole new organism from an isolated arm. This regeneration is related to asexual reproduction. Many parasitic worms reproduce asexually during their larval stages. It could be argued that the formation of monozygotic, identical twins in vertebrates, including humans, is a form of asexual reproduction.Asexual reproduction can result in the production of large numbers of progeny very speedily. However, it does not shuffle the gene pool (only mitosis is involved in cell replication with none of the opportunity for recombination of genetic material which is associated with meiosis) and so variation (on which natural selection can work) does not occur except by genetic mutations. Consequently, asexual reproduction is advantageous in a constant environment but disadvantageous if the environment is changing.
▶Sexual Reproduction
In sexual reproduction, the gene pool is shuffled by the processes of meiosis and the exchange of genetic material between two animals. In some species of Paramecium, two individual ciliophorans may come together (conjugation) and exchange genetic material before separating: here no reproduction has occurred. This phenomenon may be associated with DNA repair (see below). In many other protoctistans and in animals, genetic recombination is linked with reproduction. Sexual reproduction involves the formation, by meiosis, of gametes, that is the female egg (ovum) and the male sperm (spermatozoon). Each gamete has the haploid (half) number of chromosomes for the species. At fertilization, the egg and sperm fuse to form a zygote and the diploid (complete) number of chromosomes is restored.
Sexual reproduction is energetically very expensive because large numbers of gametes must be generated, of which only a few are used to form zygotes which grow to reproductive maturity. Even in birds and mammals where few eggs are produced, large numbers of sperm are generated and the female must expend considerable resources on the nurture of the developing embryos and infants. The advantage of sexual reproduction is the generation of variation upon which natural selection can act: such variation may be particularly advantageous in changing environments.
The evolution of sexual reproduction may be linked to another advantage associated with it: the opportunity for the repair, during the first prophase of meiosis, of damaged DNA. (Incorrect nucleotides on a DNA strand can be detected, deleted and replaced with a correct nucleotide, using the complementary DNA strand as a template.) The increase in genetic variability is a by product of this process, further amplified by linking it to reproduction. In most animal species the male and female sexes (genders) are distinct (dioecious), although hermaphroditism is found in many species, especially those which are sessile. Hermaphroditism is advantageous in that gametes from any one individual stand a chance of meeting gametes of the opposite sex from any other, neighboring individual rather than from just a proportion of such individuals. Asynchrony in the production of eggs and sperm (e.g. through the testes developing before the ovaries, i.e. protandry, as in some oyster species) or other arrangements will be necessary to avoid self-fertilization, although some tapeworms do self-fertilize.
▶Parthenogenesis
Unfertilized eggs can be stimulated into development, a process called parthenogenesis (‘virgin origins’). Artificial parthenogenesis can be induced in some species (e.g. the toad Xenopus laevis) by temperature, pH or mechanical shock to the egg: the resulting animal is often small and sterile. Natural parthenogenesis occurs in many species of animals (and plants). It is arguably a form of asexual reproduction, but can be associated with meiosis and syngamy (coming together of gametes). Some species are obligate parthenogens: in these no males are found, and offspring come from a single female parent. In some species (e.g. rotifers) environmental changes (e.g. cold, population decline) may trigger production of males and resumption of sexual reproduction. In rotifers sexually produced eggs are thick-skinned and undergo dormancy.
There are two major types of parthenogenesis: in arrhenotoky there is production of males from unfertilized eggs, while in thelytoky there is production of females from unfertilized eggs. Bees and bugs (e.g. greenflies) are examples of arrhenotokic parthenogens.
Females produce haploid eggs by meiosis. These develop into diploid females if fertilized, and into haploid males if not. In the honey-bee, the queen is the only fertile female in the hive; she is inseminated once in her life – she stores the sperm and uses it to fertilize her eggs, or does not use it. The diploid females usually grow up to be adult, sterile workers, but those few fed on royal jelly grow up to be fertile queens. The haploid males develop into the drones. There are two main forms of thelytoky. In apomixis diploid females develop from a single cell produced mitotically: there is no meiosis or syngamy. The mother passes her genome on to her daughters unchanged. An animal example is the whiptail lizard Cnemidophorus tesselatus. (This is a hybrid of two other, ancestral Cnemidophorus spp. that produce diploid eggs; males of C. tesselatus are unknown.) Thus the gene pool is not shuffled and there is no variation on which natural selection can work. In automixis (meiotic parthenogenesis) females produce haploid eggs by meiosis. Lack of males or sperm means no fertilization. Automictic species restore the egg to its diploid state by chromosome doubling devices such as pre-meiotic endomitosis. This doubles the chromosome number before meiosis I division occurs; normal meiosis divisions then proceed so that the egg has the normal diploid number of chromosomes by the end of the meiosis II division. Again this is an asexual reproduction process with no genetic recombination.
In gynogenesis (as found in the Amazon molly, Peocilia formosa) sperm are required to “stimulate” the egg but no syngamy between sperm and egg occurs and the genetic material is not fused. The eggs are diploid (produced by premeiotic endomitosis (see above), but sperm from a related species is needed to penetrate the egg. Thus the genome is passed on unchanged from generation to generation, paralleling asexual modes of reproduction (e.g. budding in tunicates and cnidarians).
▶Gametogenesis in mammals
Sperm development
Sperm are produced in the testes by a process of spermatogenesis. In some species (e.g. humans), the testes remain the same size, regardless of season; in others the testes enlarge in, and spermatogenesis is confined to, a breeding season. Germ cells are recognizable in the embryo: they colonize the gonad. In male humans, genes on the Y chromosome determine the development of testes and subsequent male morphology. (In the absence of these genes, the undifferentiated gonad develops into an ovary and the embryo develops into a female.)
In human male embryos the germ cells multiply in the highly coiled seminiferous tubules of the testis by mitosis until 5–6 months of gestation; they then become quiescent until puberty. The walls of the seminiferous tubules comprise clusters of sperm-forming cells, spermatogonia, interspersed with supporting.
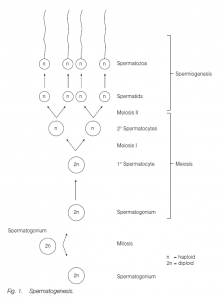
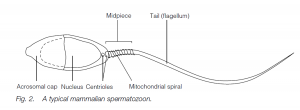
Sertoli cells (nurse cells). At puberty, gonadotropins (FSH and LH) from the anterior pituitary initiate spermatogenesis (and testosterone production by Leydig cells in the testis, leading to the development of secondary sexual characteristics). Spermatogonia undergo further mitosis (throughout adult life) to yield more spermatogonia and also primary spermatocytes which enter meiosis. The first (reduction) division of meiosis gives secondary spermatocytes, and the second meiotic division gives spermatids which further differentiate (without further division) into sperm (spermatozoa). The nucleus of the spermatid becomes condensed and most of the cytoplasm is phagocytosed by the Sertoli cells; secretory granules accumulate at the anterior end of the cells to form the acrosome (a modified lysosome). The two centrioles of the spermatid relocate behind the nucleus, the hindmost one forming the axial filament of the sperm tail which has the characteristic 9 + 2 structure of a flagellum.
A sheath may thicken and strengthen the proximal end of the tail. Mitochondria are found in the middle piece of the sperm, behind the head. Sperm in arthropods (e.g. lobsters) may lack a tail and move by pseudopodia. The sperm are released into the seminiferous tubules and then to the epididymis where maturation is completed. In men the time lapse between the primary spermatocyte and sperm release is about 74 days, maturation in the epididymis taking about 12 days. Ejaculation is via the male gonoduct – the vas deferens and the urethra.
The sperm suspension is mixed with nutritional and lubricatory secretions from various glands in the male reproductive tract (e.g. the prostate, Bowman’s gland, Cowper’s gland) to form semen. In a normal man, a 5 ml ejaculate of semen contains about 3.5 × 108 sperm. In most mammals, spermatogenesis is enhanced by lowered temperatures, as will occur when the testes are contained within the scrotum, outside the abdominal cavity: in some species the testes are withdrawn into the abdomen outside of the breeding season.
Egg development
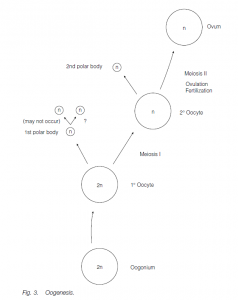
Eggs develop by oogenesis. Ovary size and activity may again be dependent on season. In many fishes, the ovaries are very large because of the large number of eggs produced; in birds and reptiles the production of yolky eggs also results in large ovaries. Germ cells (oogonia) undergoing mitotic expansion are found in the cortex of the fetal ovary. These cease mitosis and become primary oocytes between 2 and 9 months of gestation (in humans). By birth, all the germ cells are primary oocytes whose first meiotic division has been arrested at the end of prophase. Under the influence of gonadotropins following puberty, typically one primary oocyte per month (in women) completes its first meiotic division to form a secondary oocyte (containing most of the cytoplasm) and a small first polar body. The second meiotic division to form an ootid which matures to form the egg (ovum) (and a second polar body) only occurs following fertilization. Fusion of the egg and sperm nuclei produce the zygote. The polar bodies degenerate.
▶Fertilization
The gametes usually leave the body via rupture of the body wall in primitive species or, more usually, by gonoducts, the sperm duct or the oviduct. In external fertilization the eggs are shed into the water where they meet swimming sperm. In internal fertilization (associated with viviparity and/or terrestrial life) the egg is retained within the female’s body until after fertilization (as in reptiles) or even until the embryo has developed into a more or less mature infant (as in placental mammals). The sperm are introduced into the female’s body, often using an intromittent organ (e.g. the mammalian penis).
External fertilization is aided by:
- water currents carrying gametes (particularly important for sessile or sedentary animals);
- sperm carried in feeding and ventilatory currents in water;
- dense populations of individuals (e.g. mussels);
- swarming (e.g. palolo annelid worms);
- physical contact between individuals (e.g. frogs);
- production of very large numbers of gametes (100 × 106 eggs in oysters).
Internal fertilization is aided by:
- the release of sperm near to the eggs, permitting the production of fewer gametes and a saving of resources;
- the capacity for many females to store sperm after mating.
Internal fertilization involves copulation [with anatomical modifications such as a male intromittent organ (e.g. a penis) and a female receiving chamber (e.g. a vagina)] in order to transfer the sperm, usually suspended in fluid semen, although a spermatophore package is deposited in some species (e.g. the axolotl, a urodele amphibian). Some flatworms use a stylet on the penis to inject sperm beneath the partner’s skin, and several snail species ‘fire’ a calcareous dart to stimulate the partner.
Gamete lifespan is usually short, and fertilization must occur in many species within about 40 hours. Sperm and eggs of the same species ‘recognize’ each other and a reaction is initiated. Recognition can be facilitated by the release of chemical attractants into the water from the egg, specific to the species. The acrosome reaction (elucidated in sea-urchins) is the result of the recognition event:
- when a sperm touches the jelly surrounding the egg, a polysaccharide in the jelly alters the permeability of the acrosomal membrane and Ca2+ ions from the surrounding fluid enter;
- acrosomal enzymes are released which digest a way through the jelly;
- actin molecules from the acrosome polymerize, forming an acrosomal process extending to the vitelline envelope overlying the egg plasma membrane: the acrosomal process has signal molecules which link to specific receptors on the egg membranes of compatible but not of incompatible eggs;
- the vitelline membrane breaks down;
- the egg extends a fertilization cone towards the sperm;
- sperm and egg plasma membranes unite: the sperm nucleus and proximal centriole are taken into the egg.
Polyspermy (entrance of more than one sperm nucleus) is prevented by:
- rapid entry of Na+ ions, reversing the membrane polarity and preventing additional sperm binding, immediately after the entrance of the first sperm;
- a cortical reaction spreading from the entry site of the first sperm;
- the formation of a fertilization envelope (following release of Ca2+ ions and mucopolysaccharides and an osmotic inflow of water) between the plasma membrane and the vitelline envelope.
In the egg, the sperm nucleus swells to form a male pronucleus that migrates towards the female pronucleus of the egg. The pronuclei fuse in many species, or chromosomes join the mitotic spindle leading to the first division of the zygote, one centriole deriving from the sperm, the other from the egg. Calcium ion increase (which led to the cortical reaction above) activates the egg cytoplasm into metabolic activity in preparation for further growth and division.
▶Reproductive Synchrony
For fertilization, it is important that gametes are produced and then released at the same time. In most animals these events are seasonal (although not in humans). Environmental cues such as temperature, day length or tidal cycles trigger nervous or hormonal initiation of reproduction-related events such as gamete production or release. In many sessile animals gamete release into the water by one sex will stimulate gamete release by the opposite sex (e.g. in sponges); courtship behavior (e.g. in some polychaete annelids) can also act as a trigger.
▶Egg laying
In many aquatic animals, eggs merely covered by a membrane are shed into the water: survival rates are very poor so large numbers of eggs are produced. Enclosure of the egg (or groups of eggs) in a horny, leathery or gelatinous envelope enhances survival: they may further be deposited on the substratum or attached to a rock or to vegetation. Fertilization normally must occur internally before the envelope is formed (in insects the egg-case has a micropyle pore through which the sperm can enter). The terrestrial reptiles, birds and monotreme mammals have waterproof leathery or calcareous egg shells.
▶Reproductive tracts (gonoducts)
Animals which use external fertilization (e.g. starfishes) have simple, tube-like gonoducts to allow transport of gametes to gonopores for release to the outside. Animals which use internal fertilization may have male gonoduct vesicles for sperm storage prior to copulation, glands associated with the preparation of semen and a terminal portion which functions as a penis. The female gonoduct may have a chamber to receive the penis and possibly a sperm storage chamber. The female gonoduct (oviduct) may be extensible to allow the passage of large eggs, and may have glands associated with it to secrete albumin, egg envelopes or shells, or adhesive mucilage.
The vertebrate male gonoduct
In most fishes and amphibians the sperm is carried from the testis seminiferous tubules through vasa efferentia to the anterior of the opisthonephric kidney: the sperm leave via the opisthonephric duct (which also serves, not simultaneously, to drain urine from the kidney).
In amniotes (reptiles, birds and mammals), a separate, nonsegmental metanephric kidney has evolved. This kidney is drained by its own ureter. The opisthonephric duct of fishes and amphibians is now utilized solely to transport sperm and has become the vas deferens: the former opisthonephros has become the epididymis, the vasa efferentia being reduced to a tiny rete testis. Sperm are stored in the epididymis and the vas deferens, where they mature. Final acquisition of fertilizing capacity (capacitation) is only achieved after a period in the female tract in mammals. Birds lack a penis; the male brings his cloaca against the female’s cloaca during mating to facilitate internal fertilization; in mammals the penis becomes erect when aroused erotically, due to arterial dilation and consequent turgor of vascular cavities within the organ, maintained by restriction of venous return. Associated with the male gonoduct are seminal vesicles and glands which secrete components of seminal fluid.
The vertebrate female gonoduct (oviduct)
In fishes and most amphibians where fertilization is external, eggs are shed into the coelom from the ovary; the eggs enter the oviducts through ostia and pass down the oviducts where layers of jelly may be secreted around the eggs (e.g. frogspawn). The eggs may be temporarily stored in ovisacs, and are then expelled via the cloaca. The paired oviducts (or Mullerian ducts) are found in all female vertebrates: vestigial oviducts may be present in males. Unlike the situation in males, the opisthonephric kidney and its duct is not recruited for gamete transport.
In reptiles and birds (which use internal fertilization), a large egg is deposited (except in a few viviparous reptiles such as some snakes). The oviductal glands are large and secrete the albumin and shell. In birds, one of the paired oviducts is usually lost.
Viviparity is seen in marsupial and placental mammals, together with a few fishes, amphibians and reptiles (never in birds). Membranes surrounding the embryo come into close contact with a region of the oviduct (the uterus or womb in mammals) to form a placenta through which exchange of nutrients, gases, hormones, certain antibodies, waste products, etc. takes place. In placental mammals, the ostium at the top of the oviduct assumes a funnel-like shape and partially surrounds the ovary. When an egg is released from the ovary it is usually wafted into the oviduct by ciliary currents and carried down the anterior part of the oviduct, the convoluted Fallopian tube, aided by muscular contractions. The lower regions of the oviduct form the thick-walled, muscular uterus: in some species of mammals (e.g. humans) the oviducts fuse here. The neck of the uterus, the cervix, has a sphincter-like morphology, and is separated from the vagina formed from the most posterior part of the oviduct and the ventral parts of the cloaca: the vagina forms a receptacle to receive the penis during copulation
