Dextran and its production
- Dextran is the long-chain polymer of dextrose (D-glucose), used as a substrate for plasma.
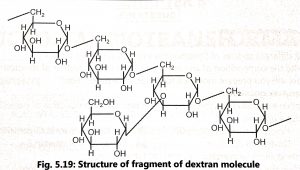
- They are slender molecules of an unbranched chain of glucose units joined by 1:6 glucosidic linkages or highly branched polymers consisting of short chains.
- It is commercially produced by Leuconostoc mesenteroides and Leuconostoc dextranicus (lactic acid bacteria).
- The fermentation medium contains sucrose as a source of carbon, peptone as a source of nitrogen and other essential growth factors.
- The microbial culture producing an enzyme ‘dextran-sucrase‘ which converts sucrose into dextran and fructose by straight-transglycosylation. Fructose is utilized by the microorganism.

- Fermentative production of dextran is similar in many respects to antibiotic production.
- Growth of the dextran-producing strain is carried out in large fermenters in media containing a high percentage of carbohydrates.
- The average molecular weight of the dextrans produced may vary with the strain used.
- Aeration is not required fermentation because aeration mainly inhibits the process. Many precautions are taken in sterilization of media, adjustment of pH, and avoidance of overheating.
- It is important to prevent the hydrolysis of sucrose to glucose and fructose during sterilization of culture dextran media.
- The dextrans produced by the fermentation have a molecular weight of 2 to 2.5 lakhs gram per mole. For clinical use, dextran has a molecular weight of upto 1 to 1.1 lakhs grams per mole.
- The fermented product is subjected to controlled hydrolysis, followed by fractionation with ethanol or acetone to produce less molecular weight dextran.
- High molecular weight dextrans may cause renal damage, allergic reactions and interfere with blood matching.
- They produce colloidal osmotic pressures that are lower than those of small molecules. Acid hydrolysis, thermal degradation, and ultrasonic bombardment techniques are used to reduce the molecular sizes of dextran.
- In acid hydrolysis, dextran is adjusted to a pH 2 and is heated at 90°C.
- In thermal degradation, the solution of dextran is heated under pressure at 160°C in the presence of sodium sulphite, to prevent oxidative degradation and calcium carbonate to neutralize acidity.
- The bombardment with ultrasonic waves splits the molecules into fragments of the same size in ultrasonic disintegration.
- The final dextran molecule is purified by solvent precipitation, adsorption, or membrane filtration.
- The selected fraction is again purified to remove reducing sugars (solvent precipitation), fractionation solvents (evaporation under reduced pressure), inorganic solvents ( ion exchange), colours (adsorption on to activated charcoal), pyrogens (adsorption on to asbestos or cellulose derivatives) and microorganisms (filtration).
- There are different official specifications for Dextran 110 injection (average molecular weight 1,10,000) to confirm that the product is suitable s a plasma substitute.
- Different chemical techniques are used to detect the amount of lead, acetone, reducing sugars, nitrogen, acid, and alkali. Biological methods confirm that the preparation is not pyrogenic, sterile, and free from proteins.
