Methods of Organ culture
The organ can be cultured in-vitro for normal development and to avoid damage of tissue, Media used for growing organ cultures are similar to tissue culture. Growing embryonic organ culture is easy than the normal organs isolated from animals. Plasma clot or watch glass method, agar gel method, raft method, the grid method, etc are mainly used for organ culture.
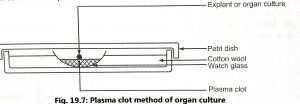
- Plasma clot or watch glass method: Explant is cultured on suitably prepared plasma clot kept in a watch glass. A plasma clot is prepared by mixing 15 drops of plasma with five drops of embryo extract. The watch glass is placed over a pad of cotton wool contained in a Petri dish (Fig. 19.7). The cotton wool is kept moist to prevent evaporation in the dish. The plate is closed with a lid, sealed with paraffin wax, incubated at 37.5°C and fresh clots have been provided every 2 to 4 days. The method is modified by using a raft of lens paper or rayon net which is used to place the tissue. The movement of the raft allows for easy transfer of tissue feeding of culture and replacement of media.
- Agar gel method: Medium containing all nutrients is solidified by using 1% agar and then kept in an embryological watch glass. Defined media with or without serum are also used to give mechanical support for organ culture. Embryonic organ or explant is transferred on the surface of agar (Fig. 19.8), sealed with paraffin wax, and subcultured into fresh agar gels after 5 to 6 days.
The different explantation techniques are used for the cultivation of pieces of fresh tissues isolated from the organism such as slide culture, carrel flask culture, roller test tube culture, etc. These techniques are modified and also use for embryo and organ culture.

Cell Lines
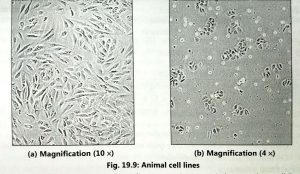
The primary culture may not be viable for a long time because the cells utilizes all nutrients of the medium. Hence, the cells are diluted with fresh medium and passed into fresh culture flask for maintaining the viability of cells. When primary culture is subcultured or transferred into fresh medium then it is called cell line (Fig. 19.9).
Subculturing is required on fresh medium at regular intervals for growth as a cell line. The first subculture gives rise to a secondary culture and so on. Number of times that the culture has been subcultured is called passage number and the number of doublings that the cell population has undergone is called generation number. Some cells of secondary cell cultures are transformed spontaneously or chemically. Such cell line or strain has the capacity for infinite survival (immortal). These cells are called continuous cell lines, cancerous cell lines or established cell lines or immortal cell lines. The continuous cell line may be discontinued by the effect of the mutation, chemical or viruses. This concept of change in a continuous cell line is called ‘in-vitro transformation’.
Cell lines are classified as finite cell lines and continuous cell lines (Table 19.1). Finite cell lines can grow only for a limited number of cell generations and they form a monolayer. These are slow-growing cells and they require 24 to 96 hours for one generation. The cell lines are anchorage-dependent and they are invariably euploid. A high serum concentration is required for the growth of finite cell lines and they have low cloning efficiency. Established cell lines are obtained from transformed cell lines or cancerous cells.
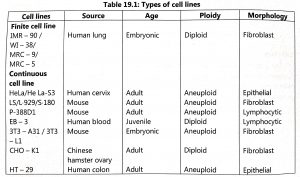
These continuous cells lines have the following characteristics:
- They have a short generation time i.e. 12 to 24 hours.
- Existence of altered ploidy i.e. heteroploidy or aneuploidy due to altered chromosome number.
- They grow has monolayer or suspension culture.
- Cloning efficiency is high and they grow well in low concentrations of serum also.
- Cell lines are anchorage-independent.
REFERENCES
https://www.sciencedirect.com/science/article/abs/pii/0014482764902319
https://www.frontiersin.org/articles/10.3389/fmed.2017.00148/full
