Protein Secretion Pathways
Bacteria and eukaryotic cells have specialized systems for exporting certain proteins (secretory proteins) to the external environment. Generally, secretory proteins are required for acquiring nutrients, cell-to-cell communication, protection, and structures that reside on the outer surface of the cell membrane. The primary impediment to the release of a secretory protein is a membrane. The processes that facilitate secretion of proteins through such a formidable barrier are similar among all organisms, although there are significant differences between organisms. For example, gram-negative and gram-positive bacteria do not have the same secretory pathways.
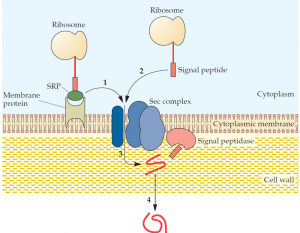
and this complex binds to a membrane protein that directs the secretory protein (1) to the Sec complex. There is also an SRP-independent pathway (2), where a signal peptide alone makes contact with the Sec complex. The secretory protein is translocated through a channel within the Sec complex (3), and the signal peptide is removed by a signal peptidase(s). Proper folding of the secretory protein occurs as it passes through the cell wall (4).
A secreted protein in gram-negative bacteria must pass through an inner membrane, a periplasmic space, and an outer membrane to exit the cell, whereas in gram-positive bacteria, secretory proteins are transported only across a single cytoplasmic membrane. In contrast, the secretory system in higher organisms is more complex. Unlike prokaryotic proteins, many eukaryotic proteins require a number of highly specific modifications, such as glycosylation, acetylation, sulfation, and phosphorylation, to produce functional secretory proteins. Some of these protein modifications and various processing steps are carried out in the endoplasmic reticulum, and others take place in the Golgi apparatus, where proteins are also sorted according to their final cellular destinations, including those that exit through the cell membrane. The property that distinguishes a protein that remains in the cytoplasm from one that is secreted is often an amino acid sequence (a signal peptide, signal sequence, leader sequence, or leader peptide) at its N terminus. In gram-positive bacteria, the signal peptide of some secretory proteins makes direct contact with a membrane-bound assembly of proteins (a secretion complex, or Sec complex) that facilitates the passage of these proteins through the membrane and their release to the external environment.
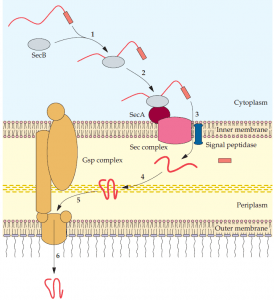
SecB attaches to the SecA protein that is part of the Sec complex of the inner membrane (2), and the secretory protein is translocated through the inner membrane (3). A signal peptidase removes the signal peptide, and the secretory protein is properly folded in the periplasm (4); the secretory protein combines with the Gsp complex (5); and it is translocated to the external environment (6).
Alternatively, for other secretory proteins, a group of proteins called a signal recognition complex binds to a signal peptide, and this combination attaches to a membrane-bound signal recognition complex receptor before making contact with the Sec complex. In both cases, the secretory protein is translocated through a channel formed by the Sec complex, and its release depends on removal of the signal peptide by a membrane-bound enzyme called a signal peptidase. Subsequently, proteins that have crossed the cytoplasmic membrane readily pass through the porous cell wall, where they encounter metal ions and other components that promote proper folding and molecular stabilization. Gram-negative bacteria have multiple pathways for the secretion of various proteins. Some of these systems (Sec-dependent pathways) use the same membrane-bound Sec complex for transmitting a secretory protein through the inner membrane into the periplasm. Collectively, the Sec dependent pathways are designated the general secretion pathway.
In these instances, a cytoplasmic protein (SecB) binds an amino acid sequence (domain) of a secretory protein that has a signal peptide. In turn, the SecB protein combines with a protein (SecA) of the membrane bound Sec complex. The secretory protein is translocated into the periplasm, and the signal peptide is removed. At this point, the secretory protein encounters various periplasmic proteins that ensure proper folding. Thereafter, Secdependent secretory proteins exit through the outer membrane by different routes. A region of some proteins is capable of forming a channel in the outer membrane that allows part of the remaining protein to be selectively extruded (autotransporter pathway). In these cases, proteolytic cleavage releases the functional portion of the protein to the external environment. Other proteins are able to pass through an outer membrane channel that is formed by a separate protein (single accessory pathway).
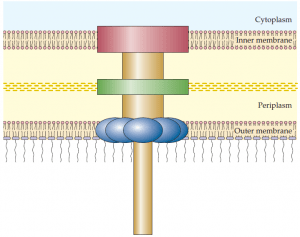
that form a continuous channel through the inner and outer membranes of gram negative bacteria. The type III secretion system is used by bacterial pathogens to secrete toxins and other proteins into plant and animal host cells. A hollow needle like protein structure extends from the bacterial surface into the host cell.
Another pathway (chaperone/usher pathway) is used by specific proteins that form fimbriae on the surface of the bacterial cell. A fourth general secretion pathway branch called the type II secretion pathway consists of a protein complex (the Gsp complex) that spans the periplasmic space and forms a channel through the outer membrane. Most secreted proteins pass through the type II pathway. In these cases, secretory proteins earmarked for the type II pathway are first transported to the periplasmic space via the Secdependent pathway, where they bind to the Gsp complex and are shunted through the outer membrane. Other Sec-dependent pathways have been found in various gram-negative bacteria. In contrast to the type II pathway, the type I and type III secretion pathways are Sec independent, and each has its own protein complex that extends from the inner to the outer membrane, forming a continuous channel from the bacterial cytoplasm to the external environment.
For example, bacterial flagellar proteins reach the outer surface of the cell by means of a type III secretion pathway. Type III secretion pathways are often used by bacterial pathogens to secrete bacterial proteins into the cytoplasm of eukaryotic host cells. Signal peptides are recognized by the Sec-independent systems but are not necessarily cleaved during the secretion process.
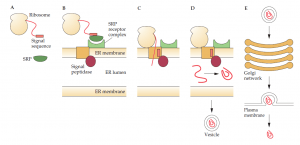
Protein secretion is basically the same in all eukaryotic organisms from yeast to plant and animal cells. Briefly, the signal sequence of a secretory protein is bound by a signal recognition particle during protein synthesis; the signal recognition particle attaches to a receptor on the membrane ofthe endoplasmic reticulum, and the secretory protein passes through a channel in the membrane as translation proceeds; a signal peptidase removes the signal sequence; and the secretory protein is released into the lumen of the endoplasmic reticulum, where it is folded and, if required, glycosylated. A vesicle containing a processed secretory protein buds off from the endoplasmic reticulum and is transported to and fuses with the cis face of the Golgi apparatus.
Additional processing, glycosylations, and posttranslation modifications take place in the Golgi stack. The secretory protein then emerges from the trans face of the Golgi apparatus enclosed in a vesicle that is transported to and fuses with the plasma membrane, where the contents are released to the external environment. In eukaryotic organisms, some proteins are secreted continuously (constitutive secretion). Others remain in vesicles (mature secretory granules near the plasma membrane and are released only after a hormone ormembrane depolarization signal is received.
