Rabies Vaccine: Introduction
Rabies Vaccine is a suspension of an appropriate strain of fixed rabies virus grown in human diploid cells (HDCV) and inactivated by β-propionilactone. The rabies vaccine is an inactivated virus vaccine prepared either in human diploid cell culture (HDCV) or in the culture of purified chick embryo cells (PCEC). HDVC may also be used for active immunization of those considered at risk (pre-exposure vaccine). In actually recommended practice two doses are administered at an interval of one month and then followed by a ‘booster dose’ one year later. Nevertheless, it is also used for post-exposure treatment in combination with human rabies immunoglobulins (HRIG). It has been duly observed that neuroparalytic and hypersensitivity reactions are intimately associated with the vaccines that are derived exclusively from either animal nerve tissues or duck-embryos; in reality, such vaccines are still used in the developing world. Most developed countries use such vaccines prepared in cell cultures, frequently HDCV. Interestingly, the usual hypersensitivity reactions are significantly less frequent with these (HDCV) preparations.
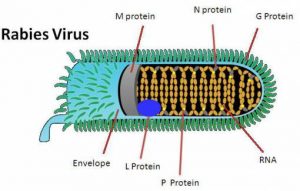
Rabies is an acute infection of mammals caused by the rabies virus. However, clinical rabies (viz., hydrophobia) is proved to be fatal in man. Infection normally has a long incubation period’ after the introduction of the virus through the bite of a rabid-animal e.g., dog, jackal, etc. If a high degree of immunity can be stimulated while the virus is slowly traversing centrally via the peripheral nerves its ultimate penetration and subsequent establishment in the brain may be prevented adequately. Modern immunotherapy procedures invariably combine both passive and active immunization in order to accomplish a rather effective high concentration as well as a high level of the antibody as rapidly as possible in vivo.
(a) Passive Immunization: Pre-exposure immunization is also recommended for such subjects who frequently handle or administer modified live rabies virus vaccines intended for animals because of the unavoidable possibility of exposure via either needle pricks or sprays, animal handlers, laboratory workers, and even veterinarians.
(b) Active Immunization: Post-exposure immunization may be inducted due to the rabies vaccination whereby antibody production commences within a span of 7-10 days and the duration of protective effect lasts for 2 years or even beyond that. It is indicated for post-exposure immunization against rabies infection.
Preparation
It may be prepared from different methods as stated below :
Method-I (Seed-Lot System): The various steps involved are :
(1) The virus used in the final vaccine represents not more than five cultures from the seed lot employed for the actual production of the vaccine on which were carried out the laboratory and clinical tests that ascertained the strain to be suitable.
(2) Animal serum may be used in the medium for the initial cell growth; however, the medium employed for maintaining the cell cultures during virus multiplication contains no protein.
(3) The concentration of serum carried over into the vaccine does not exceed one part per million.
(4) The cell culture medium may contain a suitable pH, indicator, such as phenol red, and ‘appropriate antibiotics’ usually present at the smallest effective concentrations.
(5) The virus suspension is invariably harvested on one or more occasions during incubation. In actual practice, multiple harvests from a single cell lot may be pooled and regarded as a single virus suspension.
(6) The resulting suspension is meticulously and carefully tested for identity, bacterial sterility, and above all complete freedom from extraneous viruses. In case, suspension legitimately complies with these three extremely vital tests, it is inactivated subsequently; and hence, maybe finally purified and concentrated.
(7) It is absolutely necessary to carry out the ‘amplification test’ for the residual infectious rabies virus in cell cultures derived from the same species as those employed in the production of the vaccine to confirm effective inactivation of the rabies virus. The quantum of virus used in the above test is equivalent to not less than 25 human-doses of the vaccine.
(8) Test for Live Virus: Samples of the cell-culture fluids are inoculated into mice. No live virus is detected.
(9) The resulting thoroughly tests ‘Rabies Vaccine’ is distributed aseptically into sterile containers and freeze-dried. The containers (e.g., ampoules) are sealed hermetically. The inherent residual moisture content is sufficiently low to ensure the stability of the vaccine.
(10) Potency Test: The maintenance of potency is duly verified in an ‘accelerated degradation test’ wherein the ‘vaccine’ is stored at 37°C for 4 weeks at a stretch.
Method-II (From Chick and Duck Embryos): Rabies vaccine should be prepared from such tissues that are devoid of essential the encephalitogenic material specifically. Therefore, for this particular reason, the rabies virus strains have been duly adopted to grow either in ‘chick’ or in ‘duck’ embryos. Furthermore, the viruses grown in duck-embryo are usually inactivated by β-propionilactone which process is found to be effective reasonably.
Method-III (From Brains of suckling mice, rats, rabbits, and sheep): The Rabies vaccines prepared from the brains of sucking mice, rats, rabbits, and sheep must conform to the requisite potencies by standard usual tests satisfactorily. The ‘Pharmacopoeal Method’ advocates the rabbits and sheep may be infected intracerebrally with the ‘fixed rabies virus’. Once they distinctly exhibit typical symptoms after a span of 24 hours and become paralyzed completely, they are killed and their brains are duly harvested and homogenized in NaCl injection. The resulting viruses are inactivated adequately. Phenol is the ‘chemical of choice’ and hence often preferred; however, treatment with other chemical substances, such as formaldehyde solution (formalin), β-propionilactone, or UV-light has also been equally successful. The ‘preparation’ is diluted to contain an appropriate amount of the brain material.
Rabies Vaccines Production :
Following are some of the salient features with regard to the production of Rabies Vaccines:
(1) Human subjects, immunized with rabies vaccine prepared from brains, invariably developed higher concentrations of the ‘neutralizing antibody’ in comparison to the ‘controls’ immunized with the conventional nerve-tissue vaccines.
(2) Rabies vaccine containing nerve-tissue may give rise to a serious allergic response in the brain leading to nerve cell deterioration and ultimately rendering complete paralysis.
(3) The method described in (1) and (2) above are found to be rare when the vaccines are prepared either in ‘fertile hen’ or ‘duck-eggs’. The latter is normally preferred because the yields are definitely better.
(4) β–Propionilactone is mostly used as a normal inactivated agent.
(5) In actual practice, the usage of live-avianized vaccine having attenuated virulence is seldomly done because of the difficulties encountered in producing batches of uniform and adequate potency.
(6) Active immunization against ‘rabies’ remains absolutely and mainly unsatisfactory unless and until extremely potent and highly safe inactivated vaccines are available abundantly.
(7) Proper application of ‘human diploid cells and other types of cell cultures of substrates for the growth of very high concentrations of ‘rabies virus’ is showing adequate cognizance and great promise.
(8) A plethora of ‘human diploid-cell rabies vaccines’ are mostly produced from Wilstar’s Pitman-Moore or CL-77 strain of rabies virus grown in MRC-5 human diploid cell culture. The resulting ‘vaccine virus’ is duly concentrated and then inactivated by â-propionilactone.
(9) Rabies vaccine adsorbed is prepared from the CVS Kissling / MDPH strain of rabies virus grown in a diploid cell line actually derived from fetal rhesus monkey lung cells.
(10) The vaccine virus is duly inactivated and later on concentrated by adsorption to aluminum phosphate [AlPO4].
(11) USP officially recognizes the following two variants of rabies vaccine, namely :
(a) cell-culture vaccines for intradermal usage (e.g., into the intracutaneous); and
(b) cell-culture vaccines for intramuscular injection (e.g., into the deltoid viz., triangular muscle).
(12) As a precautionary measure the ‘Rabies Vaccines’ must be administered immediately following reconstitution of the reconstituted vaccine must be discarded.
Reference
- https://www.sciencedirect.com/topics/neuroscience/rabies-vaccine
- https://www.who.int/rabies/vaccines/en/Laboratory_techniques_in_rabies_chapter27.pdf?ua=1