Contents:
Signal Amplification System
INTRODUCTION
- Biomarkers in physiological specimens serve as useful sensors for clinical diagnosis. Accurate detection of specific markers is crucial for the diagnosis of diseases, monitoring drug therapy, and patient screening.
- In-vitro immunoassays are probably the most common, simple, and relatively inexpensive serological tools used in clinical laboratories for the diagnosis and management of the disease.
- Despite continued efforts to improve the performance of immunoassays in the past three decades, there is a need for highly sensitive assays that can detect the lowest levels of disease markers with greater accuracy.
- Efforts are made towards increasing the sensitivity of immunoassays by amplifying detection signals, with implications for the development of highly sensitive diagnostic systems.
- In a conventional enzyme immunoassay, the enzyme-labeled on the antigen or antibody converts the substrate into a product.
- The product is then detected depending upon the type of substrate used. If the substrate used is a fluorescent molecule (fluorophore), then fluorescence is observed and if the substrate used is chromogenic or chemiluminescent, then change in color is measured spectrophotometrically and light emission is measured by a luminometer.
- But in an enzyme immunoassay, if the enzyme concentration is low, a weak signal will be produced and it is difficult to observe a weak signal because of background noise.
- The sensitivity of an assay is determined mainly by the detectability of the molecules. Assay sensitivity can be enhanced by amplification.
- Enzymes (such as alkaline phosphatase and horseradish peroxidase) are widely used as non-radioactive labels because they provide signal amplification through the high turnover of a substrate to detectable products.
- Further signal amplification can be introduced either by attaching multiple enzyme molecules on the target molecule, through the branched-chain DNA system, or by using enzyme-coding DNA fragments as labels which, upon expression, generate several enzyme molecules in solution.
AMPLIFICATION
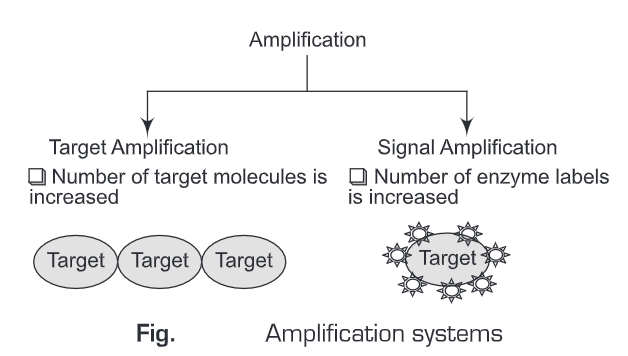
Amplification can be done in two ways. These are:
Signal Amplification
Signal Amplification means the signal is amplified by increasing the number of enzyme labels on the target molecule.
Target Amplification
Target amplification means the signal is amplified by increasing the number of target molecules. Target amplification can be achieved by PCR.
Signal Amplification Methods
In Immunoassays, different signal amplification methods are used. The most commonly used signal amplification systems are:
(a) LAB – Labeled Avidin-Biotin System
(b) BRAB – Bridged Avidin-Biotin System
(c) ABC – Avidin-Biotin Complex
(A) Soluble Enzyme
Antienzyme complexes — The sensitivity of an immunoassay can be enhanced by increasing the size of the immune complex. The size of the immune complex can be increased by either
- Direct coupling of a number of enzyme molecules to the antibody or
- Indirect coupling, i.e., enzyme-antienzyme complexes.
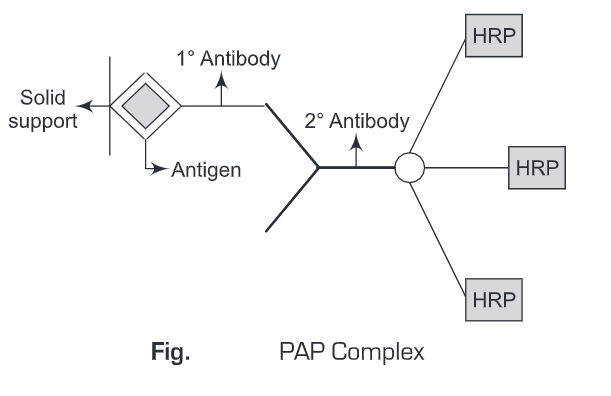
An example of an enzyme-anti enzyme complex is the peroxidase-anti peroxidase (PAP) complex. PAP (peroxidase-antiperoxidase) method was pioneered by Sternbreger in 1979. This method uses immunological sandwich amplification and the enzyme peroxidase affects the signal. The unique feature of this procedure is the enzyme/Antibody solution, the PAP immune complex.
The horseradish peroxidase enzyme, an immunogenic protein, is used to inoculate a given species, and a polyclonal immune response is generated against the enzyme. PAP complex contains three Horseradish Peroxidase (HRP) molecules. In this system, the antigen is first immobilized on a solid support. After giving a wash to remove unbound antigens, primary antibodies are added.
Again washing is given to remove unbound primary antibodies. Then secondary antibodies conjugated to three molecules of horse radish peroxidase (HRP) i.e, PAP complex , is added. A washing is given to remove any unbound PAP complex. Thereafter, substrate specific to horse radish peroxidase is added and the colored product formed is measured spectrophotometrically. Thus, in this amplification system, signal is amplified three times than the original signal.
(B) Coupled Enzyme Cascade Systems
Sensitivity of an immunoassay can be enhanced by using such an enzyme label that it induces cyclic reactions. Therefore, the enzyme label will produce a catalyst (substrate) for a second reaction or a series of reactions, and the products of such reactions will in tum act as substrate for another reaction, thus leading to a detectable product (signal).
The best example for Coupled Enzyme Cascade systems is the Alkaline Phosphatase system (ALP system). In ALP system, the enzyme alkaline phosphate catalyzes the dephosphorylation of a substrate and the product of the resulting reaction acts as a substrate for the next reaction, i.e, is capable of initiating a second reaction and thus cyclic reactions are carried out. Such cyclic reactions lead to the amplification of colored products formed at the last of the reaction, which is then measured spectrophometrically. The cyclic reaction involved in the ALP system is as follows:
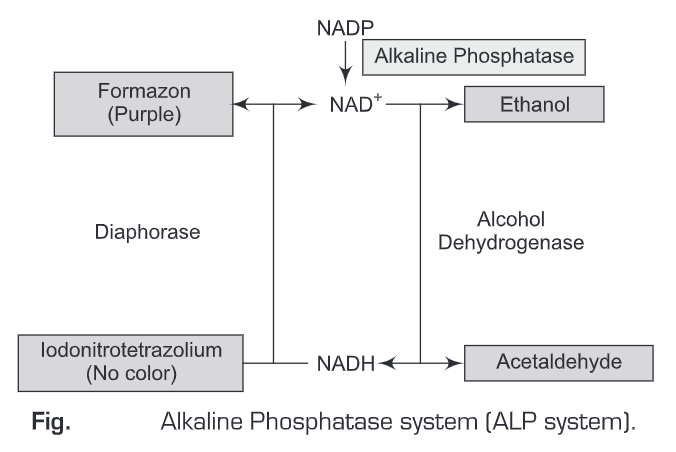
The alkaline phosphatase enzyme dephosphorylates NADP to NAD, this dephosphorylation activates an alcohol dehydrogenase (ADH) – Diaphorase redox cycle. The activated alcohol dehydrogenase oxidizes ethanol to acetaldehyde and simultaneously reduces NAD to NADH (consequence of ethanol oxidation). The second enzyme, Diaphorase now gets activated by the presence of NADH and converts the colorless substrate (INT-Iodonitrotetrazolium (INT) to a colored (purple) product, Formazan and simultaneously NADH is oxidized back to NAD. NAD can then again be used in the alcohol dehydrogenase (ADH) reaction and thereby repeating the cyclic reaction.
The net result of this cyclic reaction (NAD and NADH in the excess of alcohol dehydrogenase, diaphorase and INT) is the accumulation of the colored product, formazan. In the above cyclic reaction, each alkaline phosphatase label can produce approximately 60,000 NAD molecules per minute and NAD molecule in tum can initiate the production of around 60 molecules of the colored formazan product. Therefore, the sensitivity of this method is very high such that it can detect 0.011 molecules of alkaline phosphates.
(C) Avidin-Biotin Systems
The (strept)avidin-biotin system has been used for many years in a variety of different applications.
The Avidin-Biotin system is considered to be a versatile independent technology with broad applications in many branches of biotechnology. The biotin-avidin or biotin-streptavidin interaction has some unique characteristics that make it ideal as a general bridge system in many diverse applications which are as follows:
(a) The non-covalent interaction of avidin or streptavidin with biotin is characterized by its high affinity (Ka = 10 l/mol). This high affinity ensures that, once formed, the complex can not disturbed by changes in pH, the presence of chaotropes, or manipulations such as multiple washings after the immobilization of the complex.
(b) Avidin or streptavidin binding to biotin is specific enough to ensure that the binding is directed only to the target of interest.
(c) Both streptavidin and avidin possess four binding sites per molecule. This structural property makes possible the use of multiply biotinylated moieties (e.g., poly-biotinylated enzymes) and avidin or streptavidin to create mixtures consisting of polymers of biotinylated moieties with avidin or streptavidin. Such polymers could still have some free binding sites for biotin, thus becoming more-sensitive detection reagents in pertinent applications.
(d) Biotin is a small molecule (244.31 Da) which when introduced into biologically active macromolecules, does not affect their biological activity (in most cases), e.g., enzyme catalysis or antibody binding. Moreover, derivatized biotinylated moieties can still act as enzyme substrates or are able to bind specific antibodies. Thus, biotinylation does not usually alter many properties of the molecules.
(e) Many times avidin or streptavidin must be chemically derivatized with various organic reagents for conjugation with low- or high- molecular compounds or solid supports. As a rule, both streptavidin and avidin are exceptionally stable molecules and their biotin-binding activity can survive harsh reaction conditions and extensive derivatization. In the biotin-avidin or biotin-streptavidin system, one participating component must always be biotinylated.
The high affinity of avidin (streptavidin) for biotin in extreme conditions also provides a versatile method for signal amplification. We can exploit the avidin-biotin system in three ways for signal amplification. These are:
- LAB – Labeled Avidin-Biotin System
- BRAB – Bridged Avidin-Biotin System
- ABC – Avidin-Biotin Complex
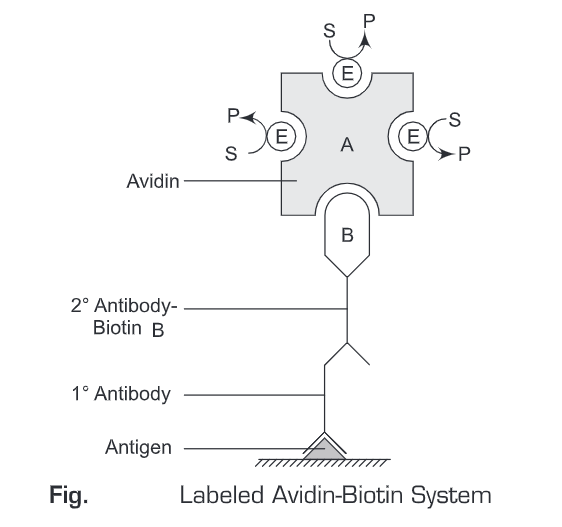
- Labeled Avidin-Biotin System —In the LAB method, we use labeled avidin molecules for the detection of antigen (or antibody). Avidin can be labeled with:
° Fluorescent molecule (fluorescein or rhodamine)
° Enzyme (Alkaline phosphatase or Horseradish peroxidase)
In the LAB method, the antigen is first immobilized on a solid support. Then a primary antibody (specific for the antigen) is added. Then a washing is given to remove any unbound antibodies. Thereafter, biotin-labeled secondary antibody is added to the reaction mixture and again a washing is given to remove any unbound antibodies. Then, avidin conjugated to an enzyme (or fluorophore) is added. A substrate specific for the enzyme (conjugated to avidin molecule) is added to the reaction mixture and incubated for 10 minutes. The colored product is then observed or measured spectrophometrically. The sensitivity of LAB method is very low.
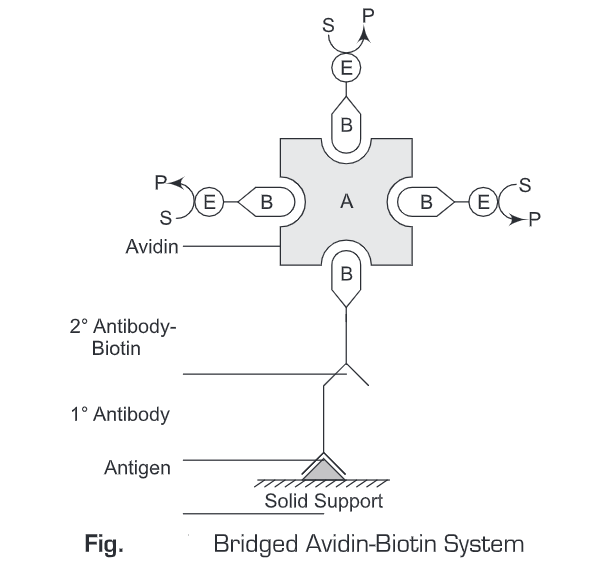
- BRAB- Bridged Avidin-Biotin System – The Bridged Avidin-Biotin System is similar to the Labeled Avidin-Biotin System with the only exception that avidin is not conjugated with an enzyme. In the Bridged Avidin-Biotin System, avidin acts as a bridge to connect the biotinylated secondary antibody and biotinylated enzyme. As avidin has four binding sites for biotin, therefore this Bridged Avidin-Biotin System permits more biotinylated enzymes to be complexed with avidin which is in turn bound to a biotin-labeled antibody. Thus, Bridged Avidin-Biotin System results in an increased amplified signal which increases the sensitivity of this method.
In Bridged Avidin-Biotin System, initially an antigen is immobilized ona solid support. Then a primary antibody (specific for the antigen) is added. Then a washing is given to remove any unbound antibodies. Thereafter, biotin labeled secondary antibody is added to the reaction mixture and again a washing is given to remove any unbound antibodies. Then, avidin is added to the reaction mixture which specifically binds to biotin. Now, biotin conjugated to enzyme (or fluorophore) are added. These biotinylated enzymes will bind to the three free binding sites on avidin molecule. Then a substrate specific for the enzyme (conjugated to avidin molecule) is added to the reaction mixture and incubated for 10 minutes. The colored product is then observed or measured spectrophometrically. The Bridged Avidin-Biotin System is more sensitive than the Labeled Avidin-Biotin System .
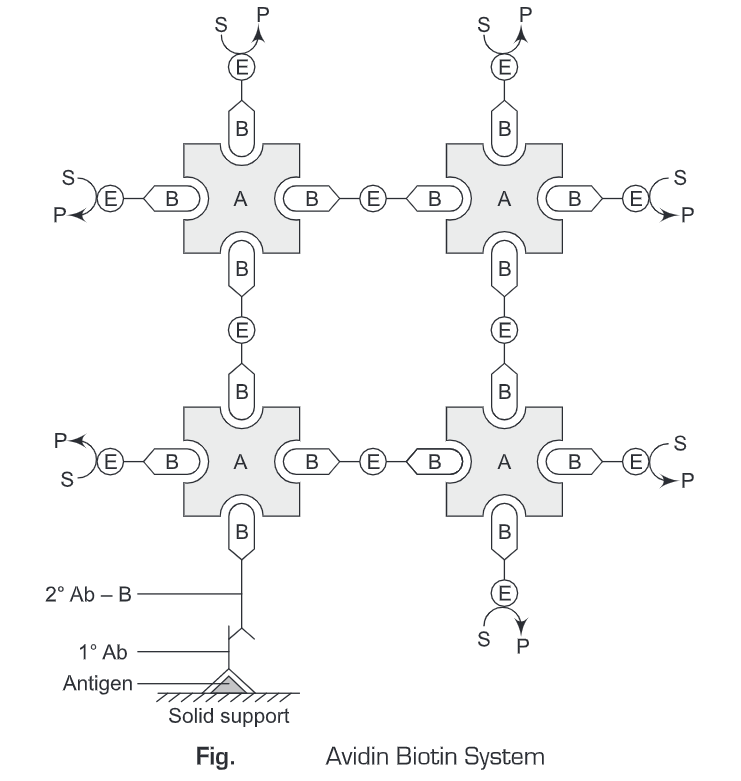
- Avidin-Biotin Complex (ABC method) – The Avidin biotin complex method is a merged form of both LAB and BRAB method. The principles of LAB and BRAB method are combined in the ABC method to increase the detection sensitivity of the system. As avidin has four binding sites for biotin, it forms a link between two or more biotin conjugates which are in turn conjugated to avidin molecules. These avidin molecules are further conjugated to biotinylated conjugates and so on. Thus, the ABC system increases the sensitivity of the detection system. This increase in the sensitivity of the ABC method is because of the increased number of enzyme molecules present in the complex.
In Avidin biotin complex System, initially, an antigen is immobilized on a solid support. Then a primary antibody (specific for the antigen) is added. Then washing is given to remove any unbound antibodies. Thereafter, biotin-labeled secondary antibody is added to the reaction mixture and again a washing is given to remove any unbound antibodies. Then an avidin-biotin complex is added to the reaction mixture. This avidin-biotin complex is conjugated to a number of enzyme molecules. Then a substrate-specific for the enzyme (conjugated to avidin-biotin complex) is added to the reaction mixture and incubated for 10 minutes. The colored product is then observed or measured spectrophotometrically. The Avidin-Biotin System is the most sensitive system, amongst the above-mentioned methods (Labeled Avidin-Biotin System and Bridged Avidin-Biotin System).
NOTE
Avidin – Avidin is synthesized in the oviduct of the hen. Avidin is 67 kDa glycoprotein consisting of 128 amino acids. Avidin is a tetrameric protein composed of all identical subunits. Each subunit is glycosylated at 17-Asparagine and has one binding site for biotin. Avidin (native or modified) is stable against heat, pH changes and chaotropic reagents. The Avidin solution is stable for weeks or even a month at 4°C. Avidin is highly soluble in water. The great affinity of Avidin for biotin (k= 10-15M) results in a number of applications in biochemistry (immunoassays, receptor and histochemical studies, bacteriophage inhibitions).
Streptavidin – Streptavidin is a neutral bacterial analog of Avidin. It is non-glycosylated and is obtained from Streptomyces avidinii and has a molecular weight of 53 kDa in its recombinant form. Streptavidin is a tetrameric protein composed of identical subunits. Each subunit binds one biotin molecule with a Kd of ~1×10-15 M. It does not show non-specific binding due to the native Avidin. It is less soluble in water. Streptavidin is preferred over avidin molecule because streptavidin has a neutral isoelectric point and does not contain carbohydrates. Such properties make streptavidin more inert in assay systems which then results in lower non-specific binding and hence greater sensitivity.
Biotin — Biotin is also known as vitamin H or vitamin By. It is present in minute amounts in every living cell. It is a water-soluble vitamin. Biotin binds very tightly to the tetrameric protein avidin (also streptavidin and neutravidin), with a dissociation constant Kd in the order of 10-15 which is one of the strongest known protein-ligand interactions, approaching the covalent bond in strength. The attachment of biotin to various chemical sites is called biotinylation and can be used as an important laboratory technique to study various processes including protein localization, protein interactions, DNA transcription and replication. For antibody labeling, biotinylation is done by attaching the amino group.