Contents:
ELISA and Its Types
INTRODUCTION
ELISA or EIA is a method widely used for measuring the concentration of a particular molecule (E.g., a hormone or drug) in a fluid such as serum or urine. The molecule is detected by antibodies that have been made against it. In simple words, in ELISA an antigen (unknown amount) is immobilized on a solid support (usually a polystyrene microtiter plate) either non-specifically (via adsorption to the surface) or specifically (via capture by another antibody specific to the same antigen, in a “sandwich” ELISA). Then a specific antibody is added over the surface so that it can bind to the antigen. This antibody is linked (conjugated) to an enzyme. Between each step, the plate is typically washed with a mild detergent solution to remove any proteins or antibodies that are not specifically bound. Then a substrate is added that the enzyme can convert to some detectable signal which indicates the quantity of antigen in the sample. Older ELISAs utilize chromogenic substrates, though newer assays employ fluorogenic substrates enabling much higher sensitivity.
Classification
ELISA can be classified into the following types:
1. Direct ELISA
- A known sample antigen is applied to a surface (often the well of a microtiter plate and the antigen adsorbs passively on incubation.
- After adsorption of antigen on the solid support, unbound antigens are washed away. A concentrated solution of non-interacting protein, such as bovine serum albumin (BSA) or casein, is added to all plate wells. This step is known as blocking because the serum proteins block non-specific adsorption of other proteins to the plate.
- Then a detection antibody specific to the antigen of interest conjugated to the enzyme is applied to all plate wells. This antibody will bind only to the immobilized antigen on the well surface, not to other serum proteins or the blocking proteins.
- The plate is washed so that the excess unbound enzyme-antibody conjugates are removed.
- The substrate is added which is then converted by the enzyme to elicit a chromogenic or fluorogenic or electrochemical signal.
- The reaction is terminated after a certain time and the result is quantified using a spectrophotometer, spectrofluorometer, or other optical/electrochemical devices.
- The antigen is diluted in a buffer, commonly a high pH (9.6) (carbonate or bicarbonate buffer or neutral phosphate-buffered saline, PBS) because the buffer contains no other proteins that might compete with the target antigen for attachment to the plastic solid phase.
- After incubation, any excess antigen is removed by a simple washing step, by flooding and emptying the wells, using a neutral buffered solution (e.g., PBS).
- Antibodies conjugated with an enzyme can now be added, and are directed specifically against antigenic sites on the solid phase-bound reagent.
- The conjugated antibodies are diluted in a buffer containing some substance that inhibits passive adsorption of protein, but that still allows immunological binding.
- Such substances either are other proteins, which are added at a high concentration to compete for the solid-phase sites with the antibody protein, or are detergents at low concentration termed blocking agents and the buffers they help formulate, which are termed blocking buffers. On incubation, antibodies bind to the antigen.
- Again, a simple washing step is then used to remove unbound antibodies (stage iv). Stage v involves the addition of a suitable substrate or substrate/chromogen combination for the particular enzyme attached to the antibodies.
- The objective is to allow the development of a color reaction through enzymatic catalysis. The reaction is allowed to progress for a defined period, after which the reaction is stopped (stage vi) by altering the pH of the system, or by adding an inhibiting reactant.
- Finally, the color is quantified by the use of a spectrophotometer reading (stage vii) at the appropriate wavelength for the color produced.
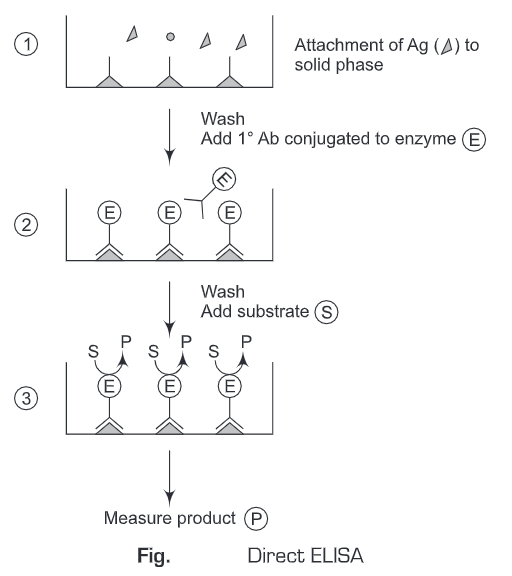
2. Indirect ELISA
- A known sample antigen is applied to a surface (often the well of a microtiter plate. The antigen adsorbs passively on incubation.
- After the adsorption of antigen on the solid support, unbound antigens are washed away. A concentrated solution of non-interacting protein, such as bovine serum albumin (BSA) or casein, is added to all plate wells. This step is known as blocking, because the serum proteins block non-specific adsorption of other proteins to the plate.
- Then an antibody specific to the antigen of interest is applied to all plate wells. This antibody will bind only to the immobilized antigen on the well surface, not to other serum proteins or the blocking proteins.
- The plate is washed, so that excess unbound antibody is removed.
- Add antibodies labeled with enzyme (conjugate) directed against the particular species in which the original antibodies were produced (anti-species).
- These antibodies labeled with an enzyme (conjugate) will bind to antibodies that are attacheded to antigen. Excess of the antibodies labeled with enzyme (conjugate) are washed away after a period of incubation.
- Substrate is added which is then converted by the enzyme to elicit a chromogenic or fluorogenic or electrochemical signal.
- The reaction is terminated after a certain time and the result is quantified using a spectrophotometer, spectrofluorometer, or other optical/electrochemical devices.
- The indirect ELISA is similar to the direct one in that the antigen is directly attached to the solid phase and then targeted by added antibodies.
- The added antibodies are not labeled with enzyme but are targeted by antibodies linked to an enzyme.
- Such antibodies are produced against the immunoglobulins of the species in which the detecting antibodies are produced and are termed anti-species conjugates.
- Thus, if the detecting antibodies were produced in rabbits, the enzyme-labeled antibodies would have to be anti-rabbit Igs in nature.
- The indirect ELISA offers the advantage that a number of antisera can be examined for binding to a given antigen by using a single anti- species conjugate.
- Such techniques have been extensively used in diagnostic applications, particularly for examining large number of samples.
- One disadvantage with this technique is the varying degree of nonspecific binding in individual sera. This tends to widen the variability in assay results and thus, increases the need to process many sera. Eg:- Indirect ELISA is used to detect the presence of serum Abs against HIV (AIDS).
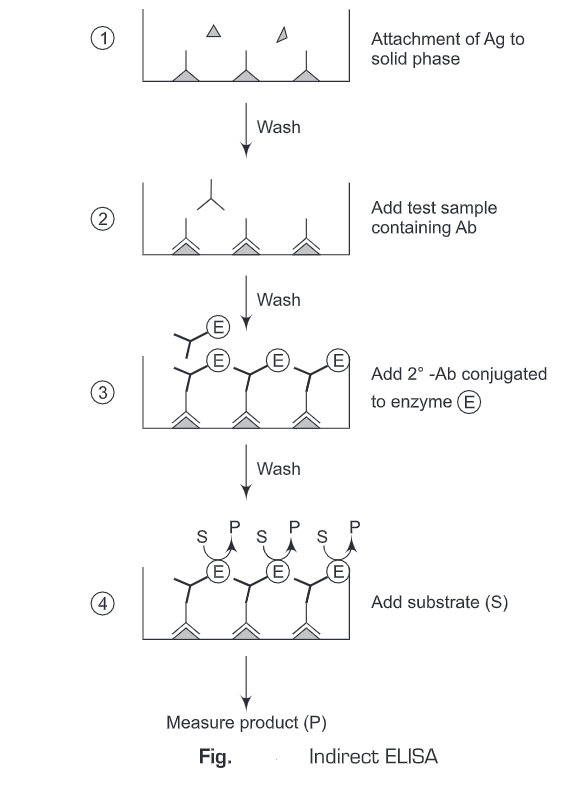
3. Sandwich ELISA
- The antigen is detected/measured
- A solution of antibody is applied to a surface (often the well of a microtiter plate. The antibody adsorbs passively on incubation.
- After the adsorption of antibodies on the solid support, unbound antibodies are washed away.
- Then an antigen-specific to antibody is added to all plate wells. The antigen will bind only to the immobilized antibodies on the well surface.
- The plate is washed, so that excess unbound antigens are removed.
- Then antibodies (conjugated with enzymes) directed against a particular antigen are added. These antibodies can be the same as used on solid-phase or from a different source (species).
- These conjugated antibodies will bind to the antigens which are attached to the antibody on the solid support. ‘Sandwich’ completes when the conjugate is incubated. Excess of the conjugated antibodies is washed away.
- Then the substrate is added which is converted by the enzyme to elicit a chromogenic/fluorogenic/ electrochemical signal.
- After a certain time, the reaction is terminated and the result is quantified using a spectrophotometer/spectrofluorometer or optical/electrochemical device.
4. Competitive ELISA
The term competition describes assays that involve the quantification of a substance by its ability to interfere with an established pretitrated system. The assays can be used for the measurement of either antibody or antigen.
- Using Antigen-Enzyme conjugate:
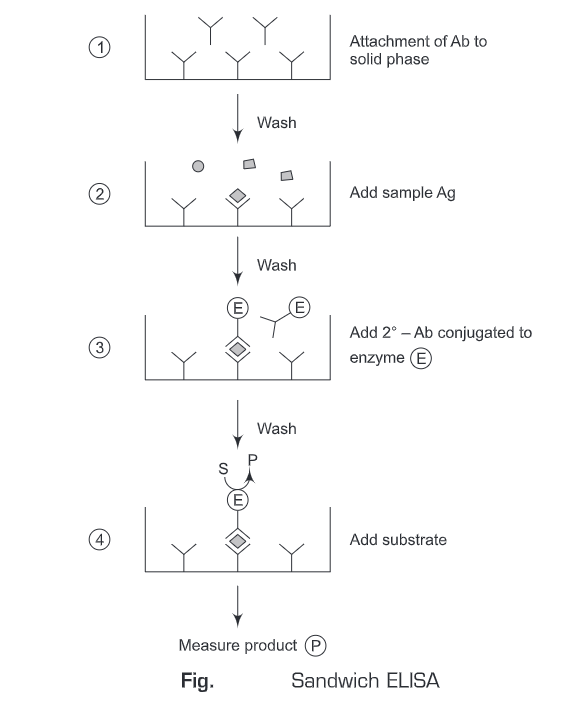
- The free antigen and antibody are incubated to form an antigen-antibody complex.
- Then the Ag-Ab complexes are added to the antigen-coated solid surface (wells). The unbound antibody-antigen complexes are washed away.
- Enzyme-linked secondary antibody against the primary antibody is then added.
- Then the substrate is added and the antigen concentration can be determined by the signal strength elicited by the enzyme-substrate reaction.
In a competitive assay, the enzyme-linked secondary antibody competes with the sample antigen which is associated with the primary antibody. The more the antigen in the sample, the fewer antibodies will be able to bind to the antigen in the well, hence “competition.” For competitive ELISA, the higher the original antigen concentration, the weaker is the detection signal.
5. Chemiluminescence
Chemiluminescence is the generation of electromagnetic radiation as light by the release of energy from a chemical reaction.
- A known sample antigen is applied to a surface (often the well of a microtiter plate and the antigen adsorbs passively on incubation.
- After adsorption of antigen on the solid support, unbound antigens are washed away. A concentrated solution of non-interacting protein, such as bovine serum albumin (BSA) or casein, is added to all plate wells. This step is known as blocking because the serum proteins block non-specific adsorption of other proteins to the plate.
- Then a detection antibody specific to the antigen of interest is applied to all plate wells. This antibody will bind only to the immobilized antigen on the well surface, not to other serum proteins or the blocking proteins.
- The plate is washed so that the excess unbound enzyme-antibody conjugates are removed.
- Luxogenic (light generating) substrate is added which is then converted by the enzyme to elicit a chromogenic signal.
- The reaction is terminated after a certain time and the result is quantified using a luminometer.
A+B — Products + light
E.g. – Oxidation of luminol by H2O, & Horseradish peroxidase produces light
Ab-HRP+Ag —> Ab-HRP-Ag —> luminol+H2O2 — Light
Chemiluminescence is more sensitive than chromogenic assays. Its detection limit can be increased 10-fold. The addition of enhancing agents, increases the detection limit by 200-fold. 5 X 10-18 moles (5 atto moles) of target Ag have been detected.
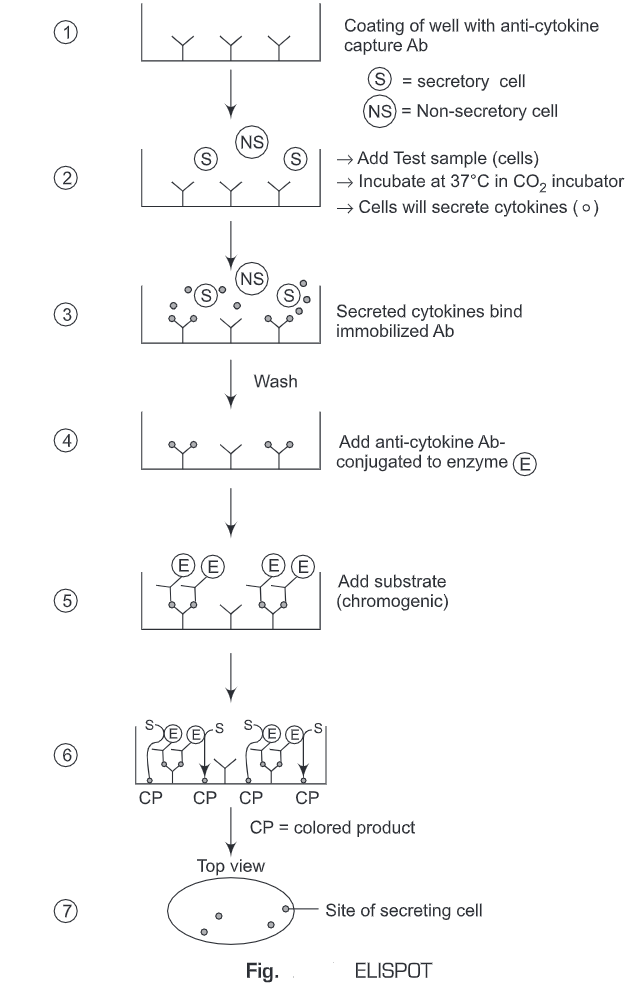
6. Enzyme-linked Immunosorbent Spot (ELISPOT) Assay
The Enzyme-linked immunosorbent spot (ELISPOT) assay is a common method for monitoring immune responses in humans and animals. It was developed by Cecil Czerkinsky in 1983. ELISPOT allows the quantitative determination of a number of cells in a population that are producing Abs specific for a given Ag or Ag for which one has a specific Ab. ELISpot assays employ the sandwich enzyme-linked immunosorbent assay (ELISA) technique.
- The immunospot plate microwell is coated with anti-cytokine capture antibodies.
- The cells (test sample) are added to the wells and incubated at 37°C in a CO2 incubator for a specific time period.
- The cells will secrete cytokines (analyte).
- During this incubation period, the immobilized antibody (anti-cytokine capture antibodies), in the immediate vicinity of the secreting cells, binds secreted cytokines (analyte).
- Unbound substances are washed away.
- A biotinylated antibody specific for the chosen analyte (cytokine) is added to the wells.
- The unbound biotinylated antibody is washed away.
- Then alkaline-phosphatase conjugated to streptavidin is added.
- The unbound enzyme is then washed away.
- The substrate solution is then added.
- A colored precipitate forms and appears as spots at the sites of cytokine localization.
- Each individual spot represents an individual cytokine-secreting cell. The spots are counted with an automated ELISPOT reader system or manually, using a stereomicroscope.
Advantages
- ELISA tests are relatively accurate tests.
- They are highly sensitive and specific.
- They do not need radioisotopes (radioactive substances) or a costly radiation counter.
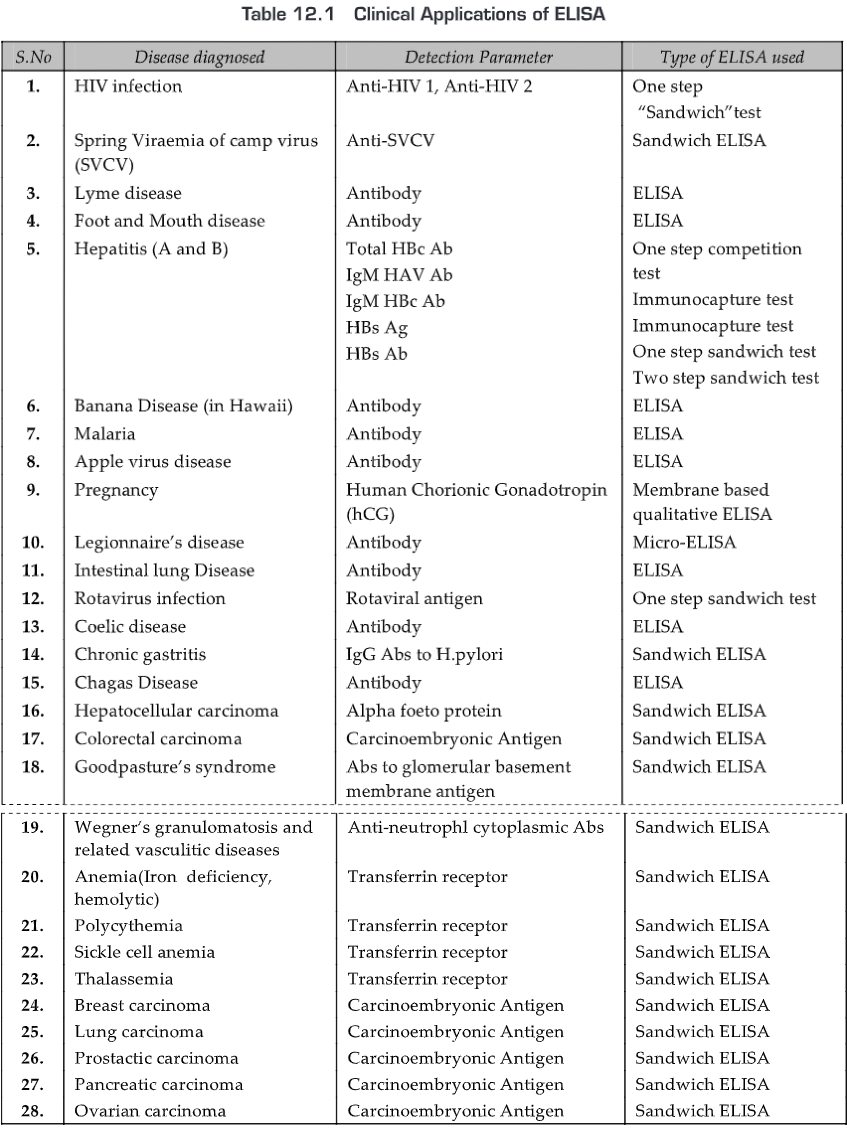
APPLICATIONS
- Immunoassays are widely used in clinical, reference and research laboratories for the detection of specific pathogens or pathogen products.
- It is desirable to use immunoassays that have high sensitivity i.e., the ability to detect very low quantities of antigen-antibody complexes.
- ELISA is amongst the most sensitive serological tests. In ELISA, Ab is labeled with enzymes for Ag detection.
- The covalent attachment of enzymes to Ab molecules decreases the amount of Ag-Ab complex required to detect a reaction.
- This increased sensitivity has been used in clinical diagnostics and biological research. As ELISA can be performed to evaluate either the presence of antigen or the presence of antibody in a sample, it is a useful tool for determining serum antibody concentrations (such as with the HIV test).
- It has also found applications in the food industry in detecting potential food allergens such as milk, peanuts, walnuts, almonds, and eggs.
- ELISA can also be used in toxicology as a rapid presumptive screen for certain classes of drugs. Thus, ELISA has numerous applications in the detection and quantitation for several Ags and Abs. Nowadays hundreds of ELISA kits are manufactured for research and human and veterinary diagnosis
Some examples:
- for screening of donated blood to detect viral contamination by
- HIV-1 and HIV-2 (presence of anti-HIV antibodies)
- hepatitis C (presence of antibodies)
- hepatitis B (testing for both antibodies and a viral antigen)
- HTLV-1 and -2 (presence of antibodies)
*For measuring hormone levels
- hCG (as a test for pregnancy)
- LH (determining the time of ovulation)
- TSH, T3 and T4 (for thyroid function)
- hormones (e.g., anabolic steroids, HGH) that may be used illicitly by athletes
*For detecting infections
- sexually transmitted agents like HIV, syphilis, and chlamydia
- hepatitis B and C
- Toxoplasma gondii
*For detecting allergens in food and house dust
*For measuring “rheumatoid factors” and other autoantibodies in autoimmune diseases like lupus erythematosus
*For measuring toxins in contaminated food
*For detecting illicit drugs, etc.,
- cocaine
- opiates
Prognosis of Diseases
Prognosis of Diseases means how we are expected to do after a disease is diagnosed. It is based on many things, including the stage of disease, kind of disease, response to treatment, and our general state of health.
1. HIV/AIDS—
- The first drugs used to treat HIV, such as AZT (zidovudine) and ddI (didanosine), have reduced the numbers of opportunistic infections and increased the life expectancy of people with AIDS, and combinations of these drugs produce even better results.
- Newer nucleoside drugs, such as d4T and 3TC, and HIV protease inhibitors (such as indinavir, saquinavir, ritonavir) and non-nucleoside reverse transcriptase inhibitors (such as efavirenz, nevirapine) are even more potent.
- In some, with effective combination treatment, the amount of virus in the blood (viral load) will decrease even to an undetectable level. Cures, however, have not been proven.
2. Small-cell lung cancer—an all-oral regimen of etoposide and cyclophosphamide is developed for use in the prognosis of this extensive disease.
3. Coronary Artery Disease—Early diagnosis and treatment can improve the prognosis for people with coronary artery disease. For angina or a minor blockage, medications and lifestyle changes may limit further damage. In serious conditions, surgical treatment such as angioplasty and stents that open the artery can help the blood flow more freely. Coronary bypass surgery can create a bypass for the artery that allows blood to flow to the heart
4. Lyme disease —
- For early cases, prompt treatment is usually curative. However, the severity and treatment of Lyme disease may be complicated due to late diagnosis, failure of antibiotic treatment, and simultaneous infection with other tick-borne diseases (co-infections) including ehrlichiosis, babesiosis, and bartonella, and immune suppression in the patient.
- If aggressive antibiotic therapy is given early, and the patient cooperates fully and sticks to the medication schedule, recovery should be complete.
- Only a small percentage of Lyme disease patients fail to respond or relapse (have recurring episodes). Most long-term effects of the disease result when diagnosis and treatment is delayed or missed.
- Co-infection with other infectious organisms spread by ticks in the same areas as Bb (babesiosis and ehrlichiosis, for instance) may be responsible for treatment failures or more severe symptoms.
5. Hepatitis A— Hepatitis A is the least serious of the common hepatitis viruses. It only has an acute (short-term) form that can last from several weeks to up to 6 months. It does not have a chronic form. Most people who have hepatitis A recover completely. Once people recover, they are immune to the hepatitis A virus. In very rare cases, hepatitis A can cause liver failure (fulminant hepatic failure) but this usually occurs in people who already have other chronic liver diseases, such as hepatitis B or C.
6. Hepatitis B—
- Hepatitis B can have an acute or chronic form. The majority (95%) of people who are infected with hepatitis B recover within 6 months and develop immunity to the virus.
- People who develop immunity are not infectious and cannot pass the virus on to others. Still, blood banks will not accept donations from people who test positive for the presence of HBV antibodies.
- About 5% of people develop a chronic form of hepatitis B. People who have chronic hepatitis B remain infectious and are considered carriers of the disease, even if they do not have any symptoms.
- Chronic hepatitis B infection significantly increases the risk for liver damage, including cirrhosis and liver cancer.
- In fact, hepatitis B is the leading cause of liver cancer worldwide. Liver disease, especially liver cancer, is the main cause of death in people with chronic hepatitis B.
7. Hepatitis C—
- Hepatitis C has an acute and chronic form but most people (75-85%) who are infected with the virus develop chronic hepatitis C.
- Chronic hepatitis C poses a risk for cirrhosis, liver cancer, or both. About 60-70% of patients with chronic hepatitis C eventually develop chronic liver disease.
- About 5-20% of patients with chronic hepatitis C develop cirrhosis over a period of 20-30 years. The longer the patient has had the infection, the greater the risk.
- Patients who have had hepatitis C for more than 60 years have a 70% chance of developing cirrhosis. Of these patients, about 4% eventually develop liver cancer. (Liver cancer rarely develops without cirrhosis first being present.) About 1-5% of people with chronic hepatitis C eventually die from cirrhosis or liver cancer.
8. Malaria — Infections with P. falciparum cause cerebral malaria resulting in mental confusion, convulsions, and coma. This prognosis is worse, and without treatment death can occur as quickly as within 24 hours.
9. Hepatocellular carcinoma—
- The classifications of hepatocellular carcinoma (HCC) currently used are based on prognostic factors obtained from studies performed years ago when most tumors were diagnosed at advanced stages and the survival rates were substantially poor.
- The Barcelona Clinic Liver Cancer (BCLC) staging classification comprises four stages that select the best candidates for the best therapies currently available.
- Early-stage (A) includes patients with asymptomatic early tumors suitable for radical therapies-resection, transplantation or percutaneous treatments.
- The intermediate stage (B) comprises patients with asymptomatic multinodular HCC. Advanced stage (C) includes patients with symptomatic tumors and/or an invasive tumoral pattern (vascular invasion/extrahepatic spread).
- Stage B and C patients may receive palliative treatments/new agents in the setting of phase II investigations or randomized controlled trials.
- End-stage disease (D) contains patients with extremely grim prognosis (Okuda stage III or PST 3-4) that should merely receive symptomatic treatment.
10. Anemia—If iron-deficiency anemia is treated by stopping the source of the blood loss, the anemia should resolve once the iron stores are repleted, and should not reoccur unless another site of bleeding arises. Pernicious anemia, caused by B12 deficiency, generally must be treated life-long with B12 injections and will reoccur if the B12 treatment is stopped. Inherited anemias, such as thalassemia, are lifelong conditions as well.
11. Sickle cell anemia—Several factors aside from genetic inheritance determine the prognosis for affected individuals. Therefore, predicting the course of the disorder based solely on genes is not possible. In general, given proper medical care, individuals with sickle cell anemia are in fairly good health most of the time. The life expectancy for these individuals has increased over the last 30 years, and many survive well into their 40s or beyond. In the United States, the average life span for men with sickle cell anemia is 40-44 years; for women, it is 46-50 years.