THEORY
DAPI (4′,6-diamino-2-phenylindole, dihydrochloride) molecules are able to penetrate cell membranes and bind to the double helix of the DNA. Cells can be easily counted, if a known volume of a fixed water sample is filtered through a membrane and, after staining the filter, the surface is investigated by an epifluorescence microscope. DAPI is a nucleic acid fluorescent stain that binds to the rich double-stranded A-T minor groove of DNA. For DAPI to enter the cell and bind DNA, cells must be penetrated and/or fixed. When DAPI is bound to double-stranded DNA, fluorescence increases about 20fold. The membranes of dead cells can penetrate. It is used to improve the visualization under a microscope of the cell or certain cell components. The stain is very stable and non-toxic for many days or longer to living cells.
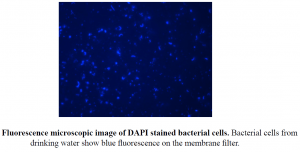
OBJECTIVE
Microbes of surface water samples
MATERIALS REQUIRED:
- Disposable gloves
- Laboratory scales
- Paraformaldehyde (PFA)
- Beaker
- Magnetic stirrer
- Phosphate buffered saline (PBS)
- Conc. NaOH solution
- Pasteur pipette
- Membrane filtration apparatus
- Polycarbonate and cellulose nitrate membrane filters (0.22 or 0.45 μm pore size)
- 50 mL Falcon tube
- Plastic Petri dishes
- Scalpel
- Pipette with pipette tips
- DAPI solution (1μg/mL)
- 80% ethanol
- Double distilled water
- Glass slide
- Coverslip
- Vectashield Mounting Medium
- Immersion oil (non-fluorescent)
- Epifluorescence microscope with a mercury lamp
- Digital camera
- Computer with adequate software
PROCEDURE
1. For the preparation of the fixative solution, dissolve 1 g PFA in 50 mL PBS. (PFA causes irritation when inhaled, therefore the use of a fume hood is recommended.) Dissolution can be aided with heating (ca. 60°C), permanent stirring, and adding some drops of cc. NaOH solution.
2. Adjust to pH to 7.0.
3. Filter the solution through a 0.22 μm pore size membrane filter. (The prepared 2% PFA solution can be stored in the fridge for one week).
4. Filter the water sample (2-50 mL, depending on the type of sample) using a polycarbonate membrane filter (slowly with occasional stirring). To help uniform cell distribution, place a 0.45 μm pore size cellulose nitrate membrane filter between the sieve of the filtration unit and the polycarbonate filter.
5. Fill the Falcon tube with fixative (PFA solution) and immerse the filter in it with sterile forceps (PFA solution must cover the entire membrane filter).
6. Incubate the filter overnight at 4°C.
7. Fill PBS into an empty Petri dish, then transfer the filter into the PBS solution for 1-2 minutes (liquid must cover the entire membrane filter).
8. Transfer the filter to another empty Petri dish and let it dry.
9. Cut a 0.5 by 0.5 cm piece from the filter with a scalpel or scissors, and pipette 30 μL DAPI solution onto its surface. From this step onwards, work in a dark place. The filter piece can be marked with a soft pencil.
10. After 2 minutes, transfer the filter into 80% ethanol for a few seconds.
11. Dip the filter paper into double distilled water for a few seconds.
12. Dry the filter.
13. Place the filter onto the surface of a glass slide, put a drop of Vectashield Mounting Medium onto the filter, and then cover with a coverslip. Cover with a paper towel and gently press the coverslip to remove any excess mounting medium.
14. Examine the slide with an epifluorescence microscope using a 100x objective and immersion oil under UV excitation (the absorption maximum of DAPI is at 358 nm, and the emission maximum is at 461 nm).
15. Record images from at least 20 different microscopic fields with a digital camera.
16. Count the cells on each picture and determine the mean values.
17. Determine the cell count for a one mL water sample based on the amount of filtered water and the size of the membrane filter field. Evaluate the variability of cell morphology.
REFERENCES
https://pubmed.ncbi.nlm.nih.gov/1725854/https://www.miltenyibiotec.com/US-en/products/dapi-staining-solution.html?countryRedirected=1
https://serc.carleton.edu/microbelife/research_methods/microscopy/index.htmlhttps://pubmed.ncbi.nlm.nih.gov/21205856/
