Introduction
The microbiological assay is a standard analytical method used to check the potency of antibiotics. It can be demonstrated by checking the inhibitory effect of antibiotics against specific kinds of microorganisms.
The microbial assay can be performed by two general methods as follows;
- Cylinder-plate method
- Turbidimetric method
Cylinder-plate method
Aim: To perform the cylinder-plate method.
Introduction:
The cylinder plate method depends on the diffusion of an antibiotic from a vertical cylinder through a solidified agar in a Petri dish. The growth of the added microorganism is prevented through the action of antibiotics around the cylinder. After incubation, the diameter of the inhibition zone is measured. The diameter depends on the concentration of the antibiotic used and its specific activity. This method has accuracy and is so used in the commercial preparation of antibiotics.
Antibiotic assay helps physicians for proper selection of antibiotics which is more effective against the causative microorganism of a particular disease.
Requirements:
- Media: Muller Hintons media for bacteria, Potato Dextrose media for fungi.
- Microbial culture: 24 hours fresh bacterial culture or fungal culture
- Glasswares: Sterile Petri dishes, inoculating wire loop, forceps, sterile test tubes, Cork Borer, Paper disc, Glass marking pencil.
- Apparatus: Autoclave, Hot air oven, Incubator.
Method:
- Prepare media as per standard procedure and subject it to sterilization in an autoclave.
- Prepare the bacterial suspension which has to be examined for antibiotic assay
- Immediately pour the sterile media into sterile Petri dishes.
- Evenly spread the bacterial culture on the surface of media with the help of a sterile swab under aseptic conditions.
- Prepare a solution of antibiotic having a concentration equal to a reference standard.
- Form the wells in the media with the help of cork-borer.
- Apply the solution of the antibiotic in the cavity. Similarly, apply reference standard into another cavity of the same Petri plate. (Note: in place of using the same plate for sample and reference material one can use a separate plate for individual substance.)
- Alternatively, instead of the formation of the cylinder, a paper disc is impregnated in the solution of antibiotic and reference substance.
- Using flame sterilized forceps, gently press each disc to the agar media.
- In a single plate, a maximum of three dilutions of reference can be used at a time along with a sample under testing.
- Incubate the plate overnight in an incubator at 37º C.
- Examine the zone of inhibition on the surface of media and around the cavity.
- Zone size is measured from the edge of the disk to the end of the clear zone.
Observation: Observe the formation of a zone of inhibition around the well of antibiotics.
Result:
Positive result: Formation of clear zone around the well indicates positive test and show zone of inhibition. It indicates that the microbes are sensitive to a given concentration of antibiotics.
Negative Result: If the zone of inhibition is not formed around the well indicates a negative result which means that microbes are resistant to a given concentration of antibiotic.
Assay of Streptomycin
Aim: To perform bioassay Streptomycin.
Introduction: Streptomycin is used as a therapeutic agent for diseases. The agar diffusion method is the most commonly used method for the assay of streptomycin.
Principle:
The Agar diffusion technique is an important technique for assessing microbial susceptibility to antibiotics. It involves the measurement of the zone of inhibition formed by the interaction between antibiotics and microorganisms. Diffusion of antibiotic solution from cylinder and surface area of inoculated agar media are the most important factors in the determination of the diameter of zone of inhibition.
Requirements:
- Bacterial culture: Bacillus subtilis
- Media: Antibiotic assay medium
- Apparatus: Sterile Petri dishes, test tubes, Pipettes, cork borer, conical flask, glass spreader.
- Instruments: Incubator, Autoclave, Hot air oven, laminar airflow, antibiotic zone reader.
Method:
- Prepare media as per standard procedure and subject it for sterilization in an autoclave
- Pour the media in the sterile Petri dishes and allow it to solidify at room temperature.
- Evenly spread B. Subrilis culture on media under aseptic condition.
- Prepare a cup or wells on the plate with the help of a sterile cork borer by keeping a sufficient distance between the wells.
- Add standard and different dilutions of antibiotics in the wells.
- Keep the plates in the refrigerator for about half-hour for appropriate diffusion of antibiotics at low temperature.
- Incubate the plates at 37°C for 24-48 hours.
- Observe the plates for the inhibition zone formed by antibiotic solution.
Observation:
Examine the zone of inhibition around the well. With the help of the zone, the reader measured the zone inhibition.
Result:
Positive result: The formation of a comparative clear zone around the well of the sample with respect to standard substance indicates a positive test. It indicates that the microbes are sensitive to a given concentration of antibiotics.
Negative Result: If a zone of inhibition is not formed around the well indicates a negative result. And no zone formation means microbes are resistant to a given concentration of antibiotics.
Assay of cyanocobalamin
Aim: To perform microbial assay of cyanocobalamin (vitamin B12).
Introduction and principle:
Vitamins are important growth factors required for the replication and growth of microorganisms. The name vitamin is obtained from vital amines as it was originally derived from substances like amines. Microorganisms are very sensitive to even a small concentration of these growth factors. Microbial assay of vitamins is based on the principle that microbes interact with vitamins and synthesized a byproduct that can be assayed by a suitable method.
Microbial assay of cyanocobalamin can be done by two methods viz.
- Titrimetric method
- Turbidimetric method
Requirement:
- Bacterial culture: Lactobacillus leichmannii ATCC 7830
- Media: Basal medium Stock solution.
- Vitamin: Cyanocobalamin (vitamin B12)
- Apparatus: Test tubes, Pipettes.
- Equipments: Autoclave, Hot air oven, Incubator, Refrigerator.
Assay method:
1. Titrimetric method:
Preparation of standard solutions:
a. Standard cyanocobalamin stock solution: 0.01 ug/ml in 25% alcohol. Store in a refrigerator for NMT 2 months.
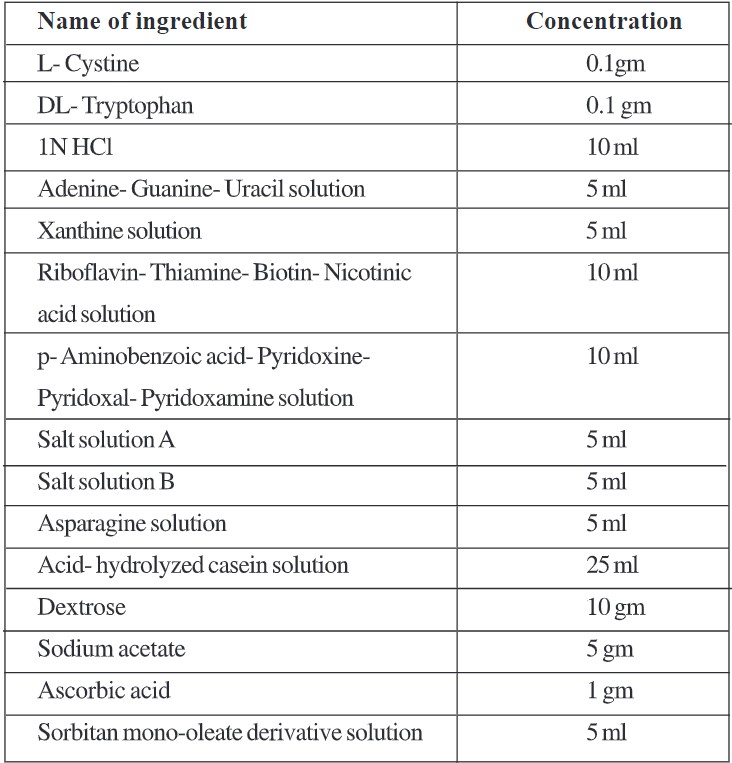
b. Basal medium stock solution:
Procedure:
- Dissolve cystine, tryptophan in HCI, and add the rest of the ingredients in order listed in the formula except dextrose, sodium acetate, ascorbic acid, and Sorbitan mono-oleate derivative solution.
- Dissolve dextrose, sodium acetate, and ascorbic acid in 100 ml water separately and add to the above mixture.
- Adjust pH 6.0 with IN NaOH.
- Add sorbitan mono-oleate derivative solution to the mixture.
- Adjust volume to 250 ml with distilled water.
c. Preparation of test solution for assay:
Dissolve the exact amount of material in water and adjust pH to 6.0 with dil. HCl or NaOH. Adjust volume with water. (Follow the procedure as given in the monograph).
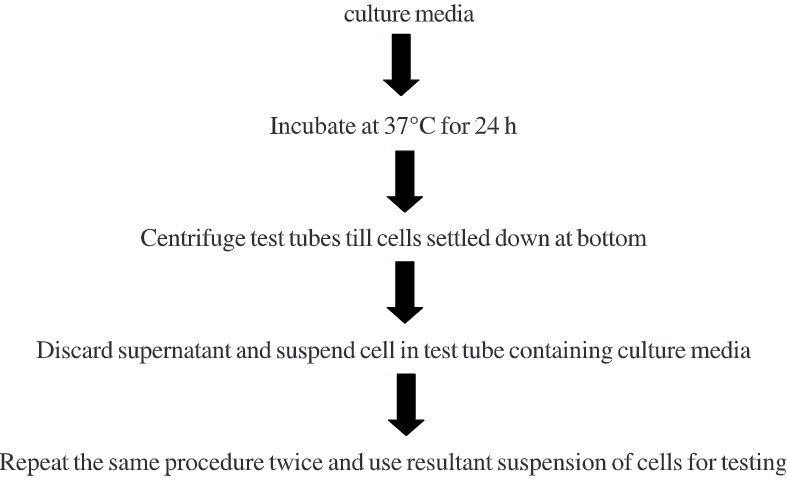
d. Inoculum preparation:
Transfer a few cells of Lactobacillus leichmannii into 2 sterile test tubes containing 10 ml.
Main Procedure:
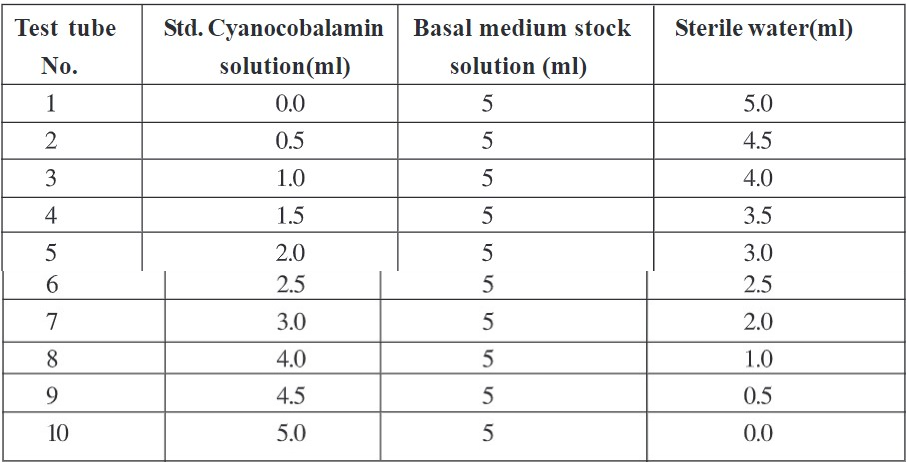
- Take 10 clean test tubes and give numbers to them as 1-10.
- Add standard cyanocobalamin solution to test tube as per volume given in table 1.
- Add 5ml of Basal medium stock solution in each test tube and adjust volume up to 10ml with sterile water.
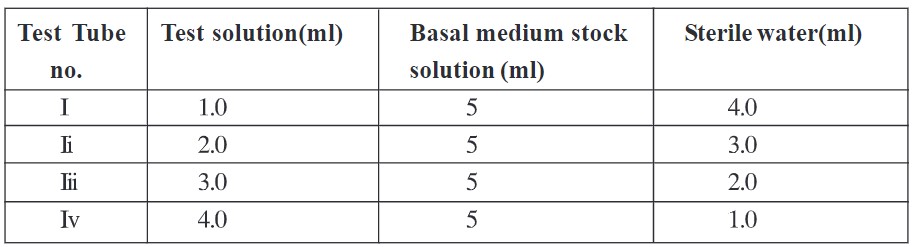
- Take 4 more test tubes and give them no. as i-iv
- To it add test solution that to be assayed in volume as given in table 2. To it add 5 ml of Basal medium stock solution and adjust volume up to 10 ml with sterile water.
- Sterilize all test tubes in an autoclave under standard conditions of temperature and pressure.
- After sterilization, keep test tubes at room temperature for cooling and then inoculated with one drop of inoculum.
- Incubate all test tubes at temperature 35°C – 37°C for 64-72 h.
- Titrate the content of each test tube with 0.05 N NaOH by using bromothymol blue as an indicator where the formation of green color is the endpoint.
- Plot the graph of volume of NaOH required (Y-axis) vs. volume of cyanocobalamin (X-axis).
- From graph compare the activity of the test sample by placing ml of NaOH required for titration of the test sample and extrapolating on the x-axis.
Result: Compare activity of test sample by using graph
Table 1 Procedure for preparation of dilutions of Std. Cyanocobalamin solution
Table 2 Procedure for preparation of dilutions of test solution
2. Turbidimetric Method
The steps involved in this method are the same as that of the titrimetric method but the only difference is that it involves the preparation of uninoculated blank tubes which contain only Basal medium-plus sterile water and in place of titration with NaOH it involves measurement of transmittance by using a colorimeter.
- Adjust transmittance to 100% at 640 nm by using the uninoculated blank tube.
- Record transmittance for all test tubes.
- Plot the graph of % transmittance (Y-axis) vs. volume of cyanocobalamin (X-axis).
- From graph compare the activity of the test sample by placing % transmittance of the test sample and extrapolating on the x-axis.
Result: Compare activity of test sample by using the graph.