Contents:
Biochemical Tests
Identification of microorganisms is done in two steps;
Step I: Examination under the microscope
Step II: Biochemical test for final confirmation.
Examination of microorganisms under microscopical examination, biochemical test plays important role in identification, classification, and confirmation of microbes. It includes various tests as given below;
- Amylase detection test
- Cellulose production test
- Urease detection test
- Hydrogen sulfide production test
- Casein hydrolysis test
- Gelatin Hydrolysis test
- Catalase test
- Pectolytic enzyme test
- Carbohydrate fermentation
- Fermentation of sugars like Glucose, lactose, or sucrose
- Mannitol fermentation
- Mannitol fermentation test by using ‘Phenol red Mannitol broth’
- Mannitol fermentation test by using “Mannitol salt agar media’
10. Indol test
-
- By using Kovacs reagent or Ehrlichs reagent
- By using Indol Spot reagent
11. Methyl Red Test
12. Vogus Proskauer Test
13. Citrate Utilization Test
Amylase detection test
Aim: To perform amylase detection test Introduction:
Amylase is an enzyme present in all living organism which plays important role in chemical process of digestion. It hydrolyzes starch into sugars by breaking down glycosidic linkage. Starch is a complex carbohydrate made up of glucose which acts as source of carbon and energy.
Principle:
This test is based on the principle that amylase results in the breaking of starch in sugars. In this test, iodine reagent is added into media which gives bluish-black color by the formation of a complex with starch. If a microorganism is able to produce enzyme amylase, it hydrolyzes starch into maltose and glucose which indicates by the disappearance of color around colonies.
Requirements:
- Bacterial culture: Nutrient broth consist of E.coli and Bacillus subtilis
- Media: Starch agar
- Chemicals: Grams iodine solution
- Apparatus: Petri dish, nichrome wire loop, dropper, glass marking pencil
- Equipments: Autoclave, incubator.
Procedure:
- Prepare the sterile starch agar plate and label it with the name of organism to be inoculated.
- Inoculate the starch plate with the organism (by single streak) under aseptic condition.
- Incubate the plate at 37°C for 48 h (bacteria) or at 25°C for 4-5 days (fungi).
- Flood the plate with grams iodine solution with a dropper for 30 seconds and drain off excess iodine.
- Observe the result.
Observation:
Observe the plates for starch hydrolysis around the streaking of each organism and check the color change of the medium,
Result:
Discoloration of iodine around colonies indicates hydrolysis of starch by amylase and vice-
versa.
Cellulase production test
Aim: To perform cellulase production test
Introduction and principle:
Cellulose is a basic structural material of plant species. Chemically it is polysaccharide of glucose residues with beta 1, 4 glycosidic linkage. Degradation of cellulose is brought by some bacterial species with the help of the enzyme cellulase. It can be used for identification of cellulase producing bacteria like Acetobacter, Rhizobium, Achromobacter, Escherichia and Sarcina.
Evidence for microbial utilization of cellulose is detected by adding hexadecyltrimethyl ammonium bromide added in media. If bacteria cause degradation of cellulose it forms clear zone around bacterial colonies.
Requirements:
- Bacterial culture: Nutrient agar plate culture for bacterial species like Bacillus cereus, Potato dextrose agar plate for fungal species like Aspergillus species.
- Apparatus: Glass rod, Petri dishes, glass marking pencil, inoculating loop
- Media: Czapek mineral salt medium (pH 7.3 + 0.3).
- Chemicals: Hexadecyltrimethyl ammonium bromide
Procedure:
- Prepared the Czapek mineral salt agar medium and sterilize in an autoclave as per standard
procedure. - Pour the media into Petri dishes under aseptic condition and labeled them with the name of the microorganism to be inoculated.
- Inoculate the respective organism in the plates.
- Keep inoculated plates in an inverted position at 35°C for 2-4 days.
- Flood the plates with 1% (v/v) aqueous solution of hexadecyltrimethylammonium bromide and observe.
Observation: Examine the plates for the formation of a clear zone around the colony.
Result: A clear zone observed around the bacterial colony of Bacillus cereus indicates the degradation of CMC by the presence of extracellular enzyme cellulase.
Urease Detection Test
Aim: To check the activity of bacteria to degrade urea by the enzyme urease.
Introduction and principle:
Urea is water soluble, nitrogenous compound which is produces in urea cycle by decarboxylation of amino acid arginine. It excreted in urine as a nitrogenous waste product. Some bacteria have ability to produced enzyme urease which breakdown the urea into ammonia and carbon dioxide. Due to formation of ammonia, pH becomes alkaline. This change in pH can be use as identification parameter for bacteria by using pH sensitive media. In this test urea broth is use which shows formation of pink color at alkaline pH.
Requirements:
- Media: Urea broth, Urea agar
- Bacterial culture: 24-48 hours bacterial culture
- Equipments: Busen burner, nichrome wire loop, test tubes, glass marking pencil
- Media preparation:
Preparation of Urea broth- Take 9.5 gm of Na2HPO4, 9.1 gm of KH2PO4, 0.1 gm of yeast extract, and 0.01 gm of phenol red. Adjust the pH of the media to 6.8 and make the preparation of 20 gm of urea.
Preparation of Urea Agar Slant
Preparation of urea agar is same like that of broth, but add solidifying agent Agar-agar. Mix thoroughly and add in the sterile test tubes. Keep the tubes in slanting position and make the urea agar slant.
Procedure:
A. Urea Agar method:
- Sterilize the nichrome wire loop on the burner.
- Inoculate the Urea agar slant with the bacterial suspension.
- Incubate the tube at 35-37°C for 2-7 days.
- Observe the development of the pink color incubation period.
B. Urea Broth method:
- Sterilize the nichrome wire loop on the burner.
- Inoculate the bacterial suspension in urea broth.
- Incubate the urea broth for 24-48 hours at 37°C
- Observe the color of the media after incubation.
Application:
The urease test is useful for the detection of different species and genus of Enterobacteriaceae like Proteus, Klebsiella, and Yersinia species. This method is useful for the detection of Cryptococcus sp. Brucella and Helicobacter pylori.
Limitation:
- Some organism shows fast splitting action while some organism shows a slow reaction of splitting.
- After long incubation times, a false positive alkaline reaction may be observed.
- Reheating of urea-containing media causes decomposition of urea.
- Urea is photosensitive and easily undergoes auto-hydrolysis.
Hydrogen sulfide production test.
Aim: To perform hydrogen sulfide production test.
Introduction and principle:
Hydrogen sulfide test is used to identify organisms that are able to reduce sulfur-containing compounds to sulfide. This conversion is possible due to the presence of cysteine amino acids in polypeptide chain. For identification of formation of hydrogen sulfide, iron containing media is use in which hydrogen sulfide form complex with iron and gives black precipitation. So, formation of black precipitation is end point detection parameter. Hydrogen sulfide test is used for identification of members of family Enterobacteriaceae.
Most suitable media for this test is Triple Sugar Iron (TSI) media which can be use as TSI slant and TSI butt. The TSI slant is preferable for aerobic microbes while TSI butt is for anaerobic growth.
Requirements:
- Culture: 24 hours trypticase soy broth culture
- Media: Triple Sugar Iron
- Equipments: Test tube, inoculating loop, glass marking pencil, burner
Procedure:
- Prepare the TSI slant and TSI butt and inoculate with test microorganisms.
- Incubate the tubes at 37°C for 18 to 48 h.
- Observe the tubes for the formation of black precipitation after incubation time.
Observation: reaction between hydrogen sulfide from bacteria and ferrous sulfate (TSI media) result in information ferrous sulfide which shows black precipitation.
Result:
The formation of black precipitation indicates a positive test and vice-versa.
Casein hydrolysis test
Aim: To perform casein hydrolysis test
Introduction and principle:
Casein hydrolysis test is use for identification of casease forming microbes. Casein is a milk protein which is responsible for the white color of the milk. Due to its larger size, it is not able to permeate from plasma membrane of bacteria. Initially it get hydrolyze into amino acid by exoenzyme casease and then transported into the cell for catabolism.
This test is performed on skim milk agar plate. Milk agar is complex media containing casein, peptone and beef extract. If microbes are able to produce enzyme casease, it forms clear zone around microbial colonies by hydrolysis of casein.
Requirements:
Media: Milk agar plate
Glassware: Petri dishes, inoculating loop, busen burner, wax marking pencil.
Culture: Bacterial culture Bacillus subtilis, Escherichia coli, Proteus vulgaris
Procedure:
- Prepare a milk agar plate.
- On one plate you may inoculate three bacterial cultures by dividing the bottom of the plate into three parts with a marker.
- Inoculate plate with microbial culture under testing by streak plate method under aseptic condition.
- Also keep the positive and negative control of test.
- Invert the plate and incubate at 35p C for 1-2 days.
Observation: Observe the formation of clear zone around colonies in comparison to positive
and negative control.
Result: Formation of clear zone around colonies of test organism indicates positive result.
Gelatin Hydrolysis test
Aim: To perform gelatin hydrolysis test
Introduction and principle:
Microorganism like Bacillus, Clostridium, Proteus, Pseudomonas and Serratia utilizes gelatin as source of carbon with the help of enzyme gelatinase. Gelatinase is extracellular proteolytic enzyme which causes hydrolysis of gelatin. Hydrolysis of gelatin takes place in two steps. In first step gelatin get hydrolyze into polypeptides and in second step polypeptides are further converted into amino acids.
This test is performed by using gelatin-containing media. Gelatin is act as a solidifying agent. If it undergoes hydrolysis, it loses its consistency which is indication of bacteria producing gelatinase. So, gelatin hydrolysis test can be used to identify and differentiation of gelatinase producing and non-producing microorganism.
Requirements:
- Media: Nutrient Gelatin media or nutrient broth + 12% gelatin
- Apparatus: Nichrome wire loop, test tubes.
- Instruments: Incubator, refrigerator.
- Microbial culture:
Positive control: Proteus vulgaris
Negative control: Enterobacter aerogenes
Test microbial suspension.
- Composition of Nutrient Gelatin medium:
- Peptone- 5.0 gm/liter
- Beef Extract- 3.0gm/liter
- Gelatin- 120.0gm/liter
Final pH- 6.8
Procedure:
- Prepare nutrient gelatin media and pour it into test tubes.
- Subject it for sterilization in an autoclave.
- Inoculate media with the bacterial suspension under testing by the stabbing method.
- Incubate the test tube at 37p C for 48 hours. Simultaneously, maintain positive and negative control.
- Remove the tubes after completion of incubation and place them in an ice bath for 30 minutes or keep them in the refrigerator at 4p C.
- Observe whether there is the hydrolysis of gelatin has occurred or not.
Observation: If media undergoes liquifiction during incubation and even remains as liquid only during refrigeration indicates hydrolysis of gelatin by gelatinase.
Result: If media undergoes liquification indicates positive result and vice-versa.
Catalase test
Aim: To perform a catalase test
Introduction and principle:
While performing aerobic respiration microbes produces hydrogen peroxide which is lethal to the microbial survive. To overcome the lethal effect of hydrogen peroxide, microbial cells secrets enzyme catalase. It causes breakdown of hydrogen peroxide into water and oxygen.
This test is carried out to check the ability of microbes to synthesize enzyme catalase. In this method, trypticase soy agar media is inoculated with test microorganisms in which hydrogen peroxide is added after incubation time. Presence of catalase is identifying by formation oxygen bubbles in media.
Requirements:
- Bacterial culture: Culture of Escherichia coli, Streptococcus lactis
- Media: Trypticase soy agar slants
- Chemical: Hydrogen peroxide
- Apparatus: Busen burner, inoculating loop, test tubes
Procedure:
- Prepare trypticase soy agar slants and subject for sterilization in autoclave.
- Inoculate the slants by using bacterial culture like Escherichia coli, Streptococcus lactis separately.
- Incubate the slant tubes at 35°C for 24h.
- After completion of incubation time, add 3-4 drops of hydrogen peroxide in the slant and observe.
Observation: interaction between enzyme catalase and hydrogen peroxide results into formation of oxygen bubbles.
Result: Release of oxygen bubbles after addition of hydrogen peroxide within one minute indicates a positive test.
Pectolytic enzyme test
Aim: To perform a pectolytic enzyme test
Introduction and principle:
Pectolytic enzymes are synthesized by plants and microorganisms. These enzymes find application in food industries and in the preparation of alcoholic beverages. Aspergillus niger produces the enzyme pectinase which results in the breakdown of pectin.
Pectinase-producing bacteria can be identified by using pectin-rich media. The addition of hexadecyltrimethylammonium bromide after incubation time results in the formation of a clear zone around bacterial colonies.
Requirements:
- Bacterial culture: Aspergillus niger
- Media: Hankins medium
- Chemicals: hexadecyltrimethylammonium bromide
- Apparatus: Sterile Petri dishes, inoculating loop, bunsen burner, glass marking pencil, pH meter.
- Composition of Hankins media:
Yeast extract-10 gm, Pectin-5.0gm, Agar- 15.0gm, Distilled water- 500ml
Method:
- Prepare Hankins medium and subject it to sterilization in an autoclave.
- Allow the medium to solidify at room temperature.
- Aseptically inoculate the plates with bacterial culture and incubate at 30°C for 3-5 days.
- Flood the plates with a 1% aqueous solution of hexadecyltrimethylammonium bromide and examine.
Observation: Examine the plates for the formation of a clear zone around the colonies of bacteria.
Result: Formation of clear zone around bacterial colonies or formation of precipitation after addition of hexadecyltrimethylammonium bromide indicates a positive result.
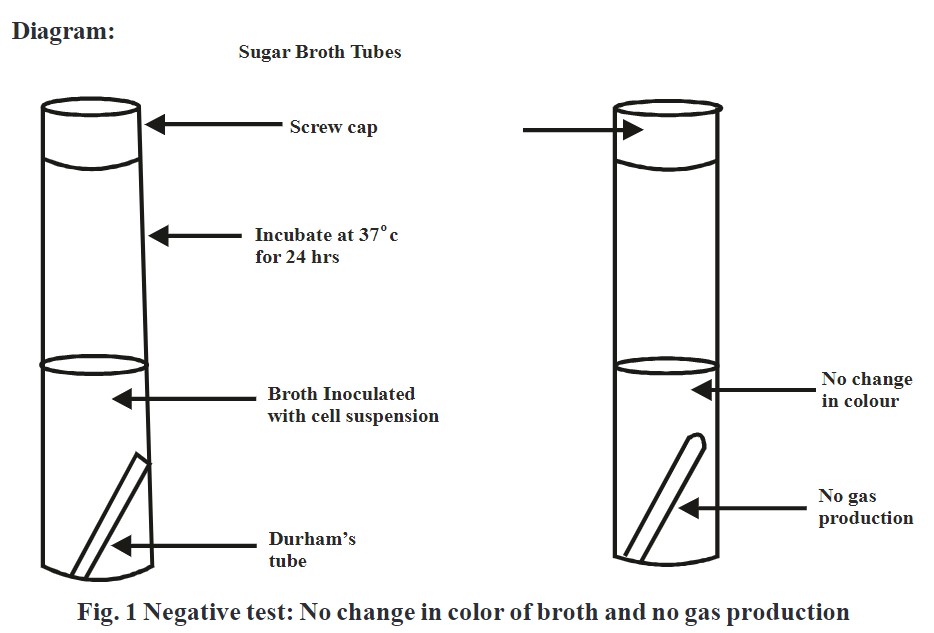
Carbohydrate fermentation:
‘Carbohydrate fermentation test’ is used to determine whether bacteria can utilize a certain carbohydrate or not. This test is based on the simple principle of production of acid and/or gas because of the fermentation of sugar like glucose, lactose, or sucrose.
It includes the following test;
Menitol Fermentation Test 1
Aim: To check the fermentation of sugars like Glucose, lactose or sucrose by bacterial cell
Principle:
Sugars gets metabolized by microorganisms aerobically as well as anaerobiaclly through different metabolic pathways. Sugar fermenting bacteria utilities carbohydrates present in liquid media and produces either acid or gas as byproduct of reaction. Formation of acid is determined by using media which shows change in color by variation in pH. Generation of air bubbles in media is indication of gas formation.
Requirements:
- Media: Nutrient broth media, Glucose broth media, Sucrose broth media, Lactose broth media.
- Indicator: Phenol red or Andrade’s indicator.
- Equipments: Test tubes, Durham tube
- Culture: Bacterial culture
- Composition of Sugar Broth Media:
Peptone-1gm
Meat Extract- 0.3gm
NaCl- 0.5gm
Distilled water- 100ml
Indicator- 0.008gm
Sugar (glucose/lactose/sucrose) – 0.5gm
Procedure:
- Prepare the media which contain three different sugars like glucose, lactose, sucrose.
- Place a Durham’s tube in inverted position into broth and subject it for sterilization in autoclave.
- Sterilize inoculating loop by heating on burner and cool.
- Aseptically transfer bacterial culture to media with the help of sterile inoculating loop.
- Incubate inoculated tubes into incubator at 35-37p C for 24h.
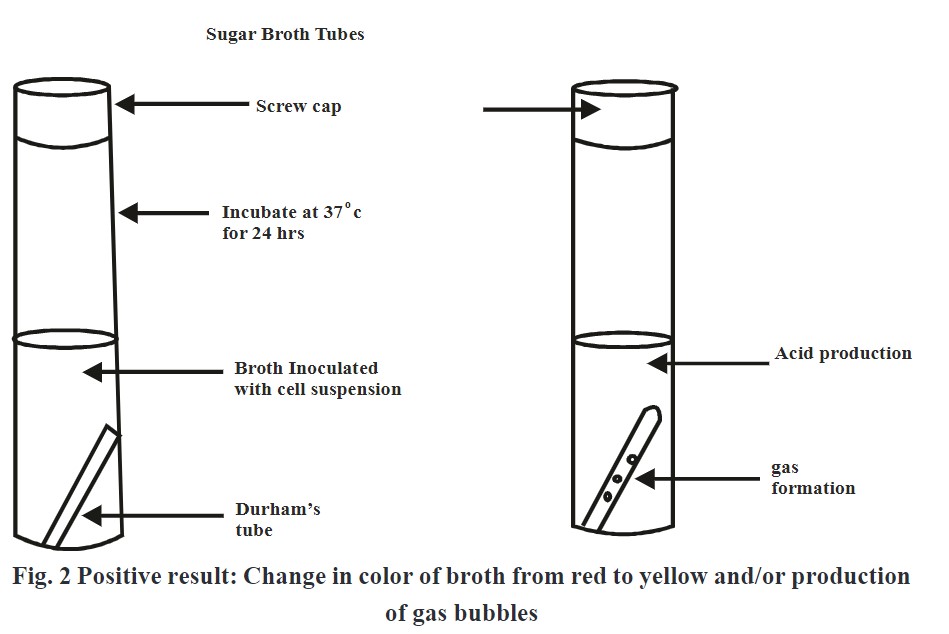
- Add phenol red indicator in each media and observe color change from red to yellow which is an indication of acid production.
- If gas bubbles get evolved in Durham’s tube indicates gas production.
Observation:
Change in color from red to yellow and/or formation of air bubbles in Durham’s tube indicates positive test.
Result:
- Yellow color of the media indicates positive test.
- Red color of the media indicates negative test.
- Yellow color and gas bubbles evolvement indicates positive test.
Manitol fermentation Test 2
Aim: To perform manitol fermentation by using broth or media
Introduction:
Manitol is also act as source of carbohydrate which is fermented by microorganism and results in the production of acid as a byproduct. It is identified by using ‘Phenol red Manitol broth’ or ‘Manitol salt agar media’ which shows change in color from red to yellow by change in pH.
Manitol fermentation test by using ‘Phenol red Manitol broth’
Requirements:
- Bacterial culture: Culture of Staphylococcus aureus
- Media: Phenol Red Mannitol broth
- Apparatus: Inoculating wire loop, test tubes
- Preparation of Phenol red Manitol broth: add 0.5-1.0% Manitol in nutrient broth.
Procedure:
- Prepare phenol red Manitol broth medium and subject it for sterilization.
- Inoculate broth with bacterial sample.
- Incubate the inoculated media in incubator at 37°C for 48 h.
- Examine any change in media.
Observation: Change in color from red to yellow indicates formation of acid by fermentation of manitol.
Result: Formation of yellow color indicates positive test and vice-versa.
Manitol fermentation test by using ‘Manitol salt agar media’
Requirements:
- Media: Mannitol salt agar
- Bacterial culture: Culture of Staphylococcus aureus
- Apparatus: Inoculating wire loop, Test tube
- Composition of Mannitol salt agar media:
- Beef extract: 1.0 gm
- Peptone: 10gm
- Sodium chloride: 75gm
- Manitol: 10gm
- Phenol red: 25mg
- Distilled water: 500ml
- Agar: 15gm
Procedure:
- Prepare Manitol salt agar media and subject it for sterilization in autoclave.
- Pour media in Petri Plates under aseptic condition and solidify.
- Inoculate media with test organism.
- Incubate the plates in incubator in an inverted position at 37p C for 48 h and observe.
Observation: If Manitol undergoes fermentation by microorganism it leads to form acid as a byproduct which causes change in pH towards acidic side. Change in pH results into formation of yellow color in media.
Result: Change in color of media from red to yellow indicates positive test and vice-versa.
Indol test
Aim: To perform indol test
Indol test is use to determine ability of certain bacteria to hydrolyze amino acid tryptophan to indol. This hydrolysis takes place in presence of enzyme tryptophanase.
Indol production test is important in the identification of enterobacteria and certain anaerobic bacteria. E.coli and Proteus vulgaris are most commonly detected by indol test.
It can be checked by two methods;
- By using Kovacs reagent or Ehrlichs reagent
- By using Indol Spot reagent
- By using Kovacs reagent or Ehrlichs reagent
1. By using Kovacs reagent or Ehrlichs reagent
Kovacs reagent reacts with indol and causes change in color from yellow to red. Development of red color takes place on the top layer of broth. Ehrlichs reagent is consist of 4 (p) -dimethylamino benzaldehyde which when reacts with indol results into development of a red colored compound.
Requirements:
- Media: Tryptophan broth
- Culture: E.coli, Proteus vulgaris
- Chemicals: Kovacs reagent
- Glassware: Test tubes, pipette
- Composition of Kovacs reagent:
- p- Dimethylamino benzaldehyde- 50.0gm
- Hydrochloric acid 37% – 250.0ml
- Amyl alcohol- 750.0ml
Procedure:
- Prepare tryptophan broth and subject it for sterilization in autoclave.
- Transfer media aseptically into sterile test tubes.
- Inoculate media with test microorganism and incubate in incubator at 37°C for 24-48 h.
- Add 0.5 ml of Kovacs reagent to the broth medium after incubation and observe.
Observation: If bacteria produces enzyme tryptophanase it causes hydrolysis of amino acid tryptophan to indol which results in development of red colored ring at top of media.
Result: Development of red color ring at the top of media indicates positive test and vice-versa.
2. By Spot Indol Test:
This test is use to determine indol by using indol spot reagent which contain cinnamaldehyde. Reaction between indol and reagent result in formation of blue green compound.
Method:
- Take drops of Indol Spot reagent on a filter paper.
- Transfer the bacterial colonies on the reagent present on filter paper.
- Observe the result immediately within 30 seconds. blue colour appear on the filter paper.
Observation: Reaction between indol and reagent result in formation of blue color within 30 seconds.
Result: Development of blue color on filter paper indicates positive results and vice-versa.
Methyl Red Test
Aim: To perform methyl red test
Introduction and principle:
Methyl red test is used to identify bacteria producing stable acids as end product from glucose source present in MR-VP media. Certain bacteria have ability to perform mixed acid fermentation of glucose and produces lactic acid, acetic acid or formic acid at the end. Formation of acid decreases pH of media nearly to 4.0-4.5. It can be determine by adding methyl red indicator which shows change in color of media from yellow to red as pH changes toward acidic side.
This test is commonly designed for the differentiation of enteric bacteria.
Requirements:
- Media: MR-VP broth
- Chemical: Methyl red indicator.
- Apparatus: Test tubes, Pipettes
Composition of MRVP broth:
- Peptone- 7.0gm
- Glucose- 5.0 gm
- Dipotassium phosphate- 5.0gm
- Distilled water- 1000ml
Method:
- Prepare MR-VP media and subject it for sterilization in autoclave.
- Aseptically pour sterilized media in two test tubes.
- Inoculate media with test microorganism and incubate in incubator at 37°C for 24-48 h.
- Add 5-6 drops of methyl red into broth after incubation time and observe.
Observation: Change in color of media from red to yellow after addition of methyl red indicator indicates that a test organism causes fermentation of glucose present in MR-VP media.
Result: development of yellow color after addition of methyl red reagent indicates positive result and vice-versa.
Vogus Proskauer Test.
Aim: To perform Vogus Proskauer Test.
Introduction:
Vogus Proskauer test is performed on MR-VP broth to identify organism from Enterobacteriaceae family like Escherichia coli, Enterobacter aerogens and Klebsiella pneumonia. Microorganism metabolize glucose in pyruvic acid which is further get metabolize into acetoin i.e. acetyl methyl carbinol as an intermediate and finally get reduced to 2, 3-butanediol. Formation of acetoin is determined by addition of alpha-naphthol and KOH which develops red color.
Requirements:
- Media: MR-VP broth (pH- 6.9)
- Reagents: Vogus-Proskauer A (Barrits reagent A), Vogus Proskauer Reagent B (Barrits reagent B)
- Preparation of Barrits Reagent A:
- Alpha-Napthol 5% – 50gm
- Absolute Ethanol- 1000ml
- Preparation of Barrits Reagent B:
- Potassium Hydroxide- 400gm
- De-ionized Water — 1000ml
Procedure:
- Prepare media and allow it to equilibrate at room temperature under aseptic condition.
- Inoculate the media with bacterial and incubate at 37°C for 24 h.
- Separate the aliquot in a clean Lest Lube and re-incubale the test tube.
- Add 6 drops of 5% alpha-naphthol reagent to the MR-VP media and mix it gently.
- Add 2 drops of 40% potassium hydroxide and mix well.
- Shake the tube gently for 1 minute.
- Expose the broth to oxygen for a color reaction to occur.
- Allow the tube to stand for 15 minutes and observe.
Observation: The formation of red color indicates that glucose get metabolize in pyruvic acid which is converted into acetoin.
Result: Formation of red color after addition of alpha-naphthol and KOH indicates positive result.
Interpretation:
- Positive Result: Glucose metabolism and form pyruvic acid which get metabolize acetoin.
- Acetoin reacts with alpha-naphthol and KOH to form red color.
- Negative Result: Glucose may get metabolism into pyruvic acid but fails to convert into acetoin. Hence; after addition of alpha-naphthol and KOH in place of formation of red color, copper color is from which indicates negative test.
Citrate Utilization Test
Aim: To perform citrate utilization test
Introduction:
Citrate utilization test is used to check the ability of organism to utilize sodium citrate as energy source. Bacteria utilize citrate as a carbon source by using enzyme citrate permease which converts citrate into pyruvate. This test is performed by using Simmonds citrate agar media containing citrate as carbon source and ammonium phosphate as nitrogen source. When bacteria metabolize citrate by using enzyme simultaneously ammonium salt gets convert into ammonia. Formation of ammonia increases pH towards alkaline side which can be determine by using bromothymol blue as indicator which turns medium from green to blue.
Requirements:
- Media: Simmonds citrate agar media
- Apparatus: Test tubes, Pipettes
- Instrument: Incubator
Procedure:
- Prepare Simmonds citrate agar slant.
- Streak the slant with test bacteria and incubate at 37°C for 4-6 days.
- Observe color change after incubation period.
Observation: Formation of blue color at surface of slant indicates that bacteria utilizes citrate as a carbon source,
Result: Formation of blue color at top of slants indicates positive result and vice versa.
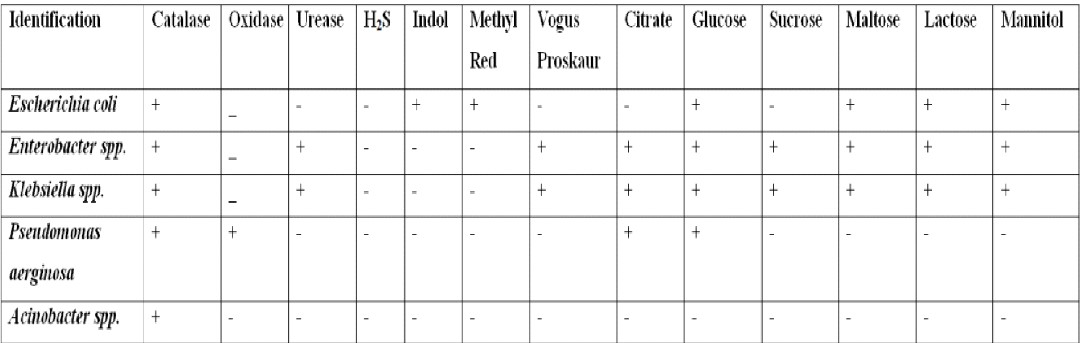
Identification of bacteria by biochemical test: