Protection of DNA Fragments
Donor genetic sequences, either genomic or cDNA, must be protected from degradation and transported through the cloning of vectors to appropriate host cells. Cloning vectors are DNA lengths that generally have three properties: (1) unique recognition sequences, (2) selectable visible markers, and (3) can be replicated. The three types of clones of vectors are (1) bacterial plasmids, (2) bacteriophage chromosomes, and (3) cosmids:
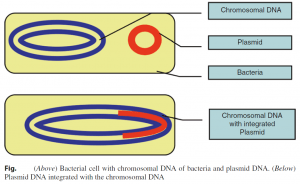
1. Plasmid vectors often contain antibiotic resistance genes and genes that control their transmission from cell to cell during the conjugation process. Most plasmids contain a polylinker or multiple cloning site (MCS), which is a short region containing a number of commonly used restriction sites, allowing for easy insertion of DNA fragments at this location. Plasmid replication may be either linked to the replication of the host chromosome “stringent plasmid” or independent of the host chromosome “relaxed plasmid.” The number of relaxed plasmids per host cell increased by the technique known as amplification.
2. Bacteriophage (or phage) vector is a virus that may infect bacteria. Bacteriophage vectors have linear double-stranded DNA molecules flanked by complementary single-stranded sequences of bases known as “cos sites.” Cos sites can bind to one another, making the phage chromosome circular. Phage particles are injecting their DNA into host bacterial cells. Phage DNA immediately directs the host bacterial cells to synthesise new phage particles in the lytic process or to become relatively inert through incorporation into the host chromosome in the lysogenic process.
3. Shuttle vectors have both yeast and bacterial replication origins and can therefore be maintained in both cell types. This property has allowed genes to be identified within the yeast cell itself. In this system, the DNA library of yeast is produced and propagated in E. Coli cells, man. When sufficient plasmid DNA is available, these plasmids are isolated from the E. Coli cells have been introduced into yeast cells with known mutations. Donor DNA fragments of approximately 4000–20,000 base pairs may be incorporated into the plasmid or phage vector, respectively.
4. Cosmid vectors are hybrid vectors consisting of a phage cos site incorporated into a plasmid molecule. Cosmid vectors may accept donor DNA fragrances of 35,000–45,000 base pairs in length. The same endonuclease then linearizes the cosmid in such a way as to leave the cosmic site intact. Both the fragments of the cosmid and the donor are mixed and allowed to bind. Some ligation products consist of a donor fragment flanked by two cosmids and therefore have two cosmic sites. A sequence located between two cos sites may be packaged into a phage particle ready to infect a host cell. As a result, the donor DNA can be inserted into the host cell through the infection process.
5. The episome is a plasmid that can be integrated into the chromosome DNA of the host organism. It can therefore remain intact for a long time, be duplicated with every cell division of the host, and become a basic part of its genetic makeup. This term is no longer commonly used for plasmids, since it is now clear that a region of chromosome homology, such as a transposon, makes a plasmid into an episome. In mammalian systems, the term episome refers to a circular DNA (such as a viral genome) that is maintained by non-covalent bonding to the host cell chromosome.
6. F-plasmid, also known fertility as F-plasmid or F-factor , is found in bacteria that allow bacterial conjugation (where genetic information is exchanged) between different bacterial cells. A bacterial cell is described as F+ (male) when it contains the plasmid and F− (female) when it does not. The F-plasmid is also an episome and can be integrated into the circular genome of the cell; in this instance, the cell would be described as Hfr. When the F+ cell conjugates with the F− cell, the result is two F+ cells, both capable of further transmitting the plasmid by conjugation. In the case of Hfr, the result is one Hfr and one F− cells. F-plasmid has also been engineered to contain inserted foreign DNA called fosmid.
7. Fosmids are similar to cosmids but are based on F-plasmid bacteria. The cloning vector is limited, as the host (usually E. coli) can contain only one fosmid molecule. Low copy number provides higher stability than comparable high copy number cosmids. Fosmid clones have been used to help assess the accuracy of the Public Human Genome Sequence.
8. R-plasmid, resistance plasmid , is a conjugative factor in bacterial cells that promotes resistance to agents such as antibiotics, metal ions, ultraviolet radiation, and bacteriophages.
9. Bacterial artificial chromosome (BAC) is a DNA construct based on a fertility plasmid (or F-plasmid) used for transformation and cloning of bacteria, usually E. coli. F-plasmids play a crucial role because they contain partition genes that promote the equal distribution of plasmids after bacterial cell division. The usual insert size of the bacterial artificial chromosome is 150 kbp, ranging from 100 to 300 kbp. A similar clonation vector, known as the PAC, was also produced from the P1-plasmid bacteria.
BACs are often used to sequencing the genetic code of organisms in genome projects, such as the Human Genome Project. A short piece of the DNA of the organism is amplified as an insert in the BACs and then sequenced. The sequenced parts are finally rearranged, resulting in the genomic sequence of the organism. BACs can carry both genes and various promoter sequences, which can often show the true level of expression of the genes. They are transferred to organisms by electroporation / transformation or by transfection with an appropriate virus or microinjection. BACs can also be used to detect genes or large sequences of interest and then used to map them to the human chromosome using BAC arrays.
REFERENCES:
