CELL IMMOBILISATION IN ETHANOL FERMENTATION
THEORY
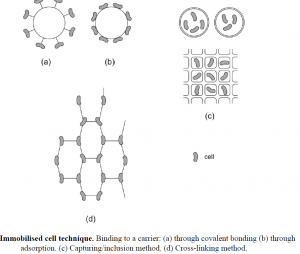
Ethanol production in the industry is carried out by Saccharomyces cerevisiae cells. The advantages of immobilized cell techniques are: higher cell density, microbial biomass can be reused multiple times, the continuous fermentation process is achievable, there is less possibility of contamination during the process and the product can be easily purified after fermentation. There are three major types of immobilized cell techniques: binding to carriers, the cross-linking method, and the capturing method. In the first case, cells or enzymes are bound to a solid carrier through adsorption, ionic or covalent bonding.
Carriers can be water-insoluble polysaccharides (e.g. cellulose, dextran, agarose), proteins (e.g. gelatine, albumin), synthetic polymers (e.g. ion exchange resins, polyvinyl chloride), and inorganic compounds (e.g. quartz). In the cross-linking method, reagents with two or more functional groups (e.g. glutaraldehyde) react with the cells. In the capturing/ inclusion method, cells are enclosed in a polymer material (e.g. alginate, polyacrylamide).
OBJECTIVE
Saccharomyces cerevisiae cell suspension (about 25 g wet cell biomass)
REQUIREMENTS
- pipettes, sterile pipette tips
- glucose broth (see Appendix)
- malt extract broth (see Appendix)
- 50 mL 4 w/w% alginate solution
- 50 mL 0.05M CaCl2 solution (pH 6-8)
- Sterile Pasteur pipette
- K-dichromate solution
- test tubes
- 37°C water bath
- Incubator
PROCEDURE
1. Mix 50 mL Saccharomyces cerevisiae cell suspension with 4% alginate solution under sterile conditions.
2. Drip the volume of this mixture into 50 ml 0.15M CaCl2 solution at room temperature. Drops of
alginate will form small balls (capturing S. cerevisiae cells inside) in the CaCl2 solution.
3. Let it solidify for 1 hour at 20-22°C, and then stabilize with an overnight incubation at 4°C.
4. Remove the CaCl2 solution and add glucose/malt extract broth the next morning.
5. Incubate at 28°C for one week without agitation. Check the clarity of the broth.
6. The produced alcohol can be detected with diluted (H2SO4) acidified potassium dichromate: add approximately 2 mL of fermentation broth to a test tube and add 6-8 drops of potassium dichromate solution into the tube.
7. Evaluate your data: in the case of a positive reaction, the potassium dichromate oxidizes alcohol to carbonic acid (through aldehyde), while the oxidation state of chromium is reduced from +6 (brown color) to +3 (green color). Examine the color change of the solution.
8. Compare the results of the two different fermentation media.
