Cell Wall Staining: Principle and Procedure
Cell wall is a rigid structure, the outer covering and is responsible for the bacterial cell shape. The cell wall has less affinity for the stains. The use of mordant is required which increases the affinity of the stain towards the cell wall and increases the thickness of the cell wall. Based on cell wall composition bacteria divides into two types: Gram-positive and Gram-negative bacteria. The Gram-positive cell wall consists of a single 20 – 80 nm thick homogenous peptidoglycan (called murein or mucopeptide) layer with teichoic acids outside the plasma membrane. Gram negative cell wall contains 2 – 7 nm peptidoglycan surrounded by 7 – 8 nm thick outer membrane made up of phospholipids and lipopolysaccharides
There are various staining techniques used to stain cell wall like Chance’s method, Ringen et al method and Dyar’s method.
(A) Chance’s Method:
Principle:
Cell wall and cytoplasm are acidic in nature where cell wall is more acidic than cytoplasm. So, when we apply the first stain that is 0.5% new fuchsin which is the basic stain, it stains the cytoplasm as well as the cell wall. Congo red is a selective decolourising agent it selectively decolourises the less acidic portion that is cytoplasm. Hence, cell wall remains stained by 0.5% New fuchsin stain and cytoplasm appears colourless.
Procedure:
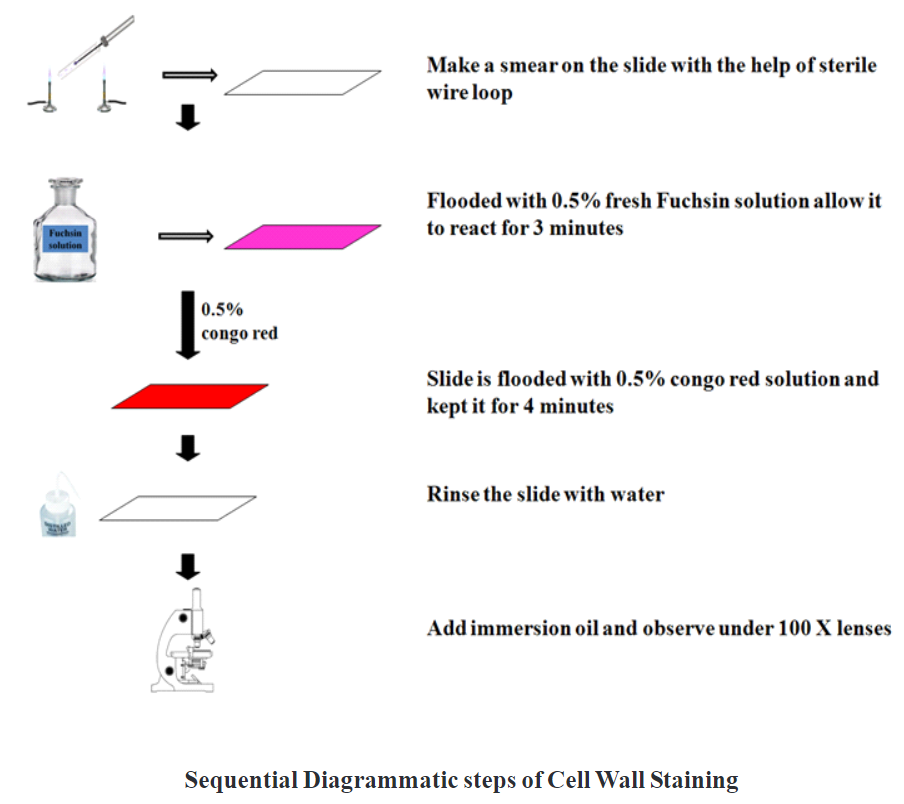
1. Take clean and grease free slide.
2. Prepare a smear of bacterial suspension using sterile wire loop.
3. Air dry the smear and heat fix.
4. Flood the smear with 0.5% New fuchsin solution and allow to react for 3 minutes.
5. After 3 minutes excess stain drains out and the slide flood with 0.5% Congo red solution and kept for 4 minutes.
6. Wash the slide gently with water.
7. Air dry and observe under oil immersion lens.
Observation:
Examine the slide microscopically using the oil immersion objective.
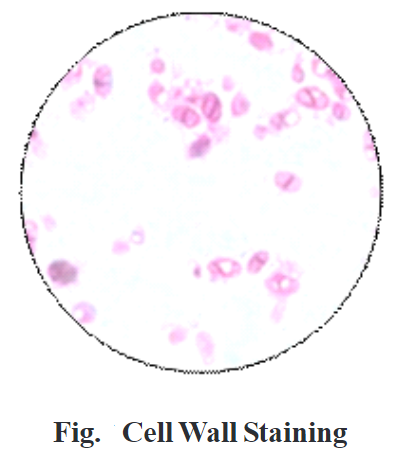
Result:
Under the oil immersion objective, we can observe pink-colored cell wall and colorless cytoplasm.
Application:
Cell wall staining helps to study the cell wall of Gram-positive as well as Gram-negative organisms due to their different cell wall chemical composition.
(B) Ringen et al Method:
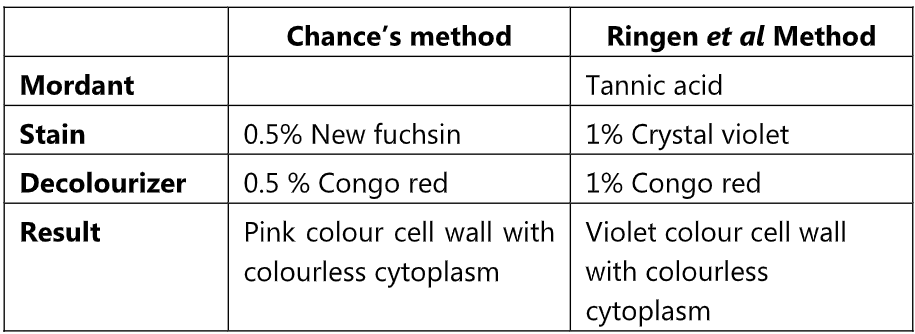
Principle of this method is like Chance’s method. In Ringen et al method tannic acid is used as mordant which increases the thickness of the cell wall and the affinity of the Crystal violet stain towards the cell wall.
Comparison of cell wall staining by Chance’s method and Ringen et al Method
(C) Dyar’s Method:
Principle:
Cetylpyridinium chloride is a cationic compound. When Cetylpyridinium chloride encounters water, positively charged Cetylpyridinium and negatively chloride ions are formed. Positively charged ions get attached to negatively charged bacterial cell surface and covered with positive ions. Congo red is acidic stain which is negatively charged stains the cell wall by contacting the positive ions on the surface of bacterial cell red in colour. Methylene blue stains the cytoplasm blue in colour. Under the oil immersion lens, we can easily observe the red coloured cell wall and blue coloured cytoplasm.
Procedure:
1. Prepare a smear on clean and grease free slide using sterile wire loop.
2. Air dry and heat fix the slide.
3. Add 3 to 4 drops of 0.35% of Cetylpyridinium chloride solution on the smear.
4. Mix a drop of 0.5% Congo red with Cetylpyridinium chloride by moving the slide slowly.
5. Keep the slide for 2 minutes.
6. Wash the slide with water and add few drops of methylene blue for 20 seconds.
7. Wash the slide with water.
8. Observe under oil immersion lens.
