Contents:
Criteria for Fermentor Designing
Introduction
- The design and mode of operation of a fermentor mainly depend on the production organism, the optimal operating condition required for target product formation, product value, and scale of production.
- The design also takes into consideration the capital investment and running cost.
- Large volume and low-value products like alcoholic beverages need simple fermentors and do not need aseptic conditions.
- High-value and low-volume products require a more elaborate system of operation and aseptic conditions. Bioreactors differ from conventional chemical reactors in that they support and control biological entities.
- As such, bioreactor systems must be designed to provide a higher degree of control over process upsets and contaminations, since the organisms are more sensitive and less stable than Chemicals. Biological organisms, by their nature, will mutate, which may alter the biochemistry of the bioreaction or the physical properties of the organism.
a) The concentrations of starting materials (substrates) and products in the reaction mixture are frequently low: both the substrates and the products may inhibit the process. Cell growth, the structure of intracellular enzymes, and product formation depend on the nutritional needs of the cell (salts, oxygen) and the maintenance of optimum biological conditions (temperature, concentration of reactants, and pH) within narrow limits.
b) Certain substances inhibitor’s effectors, precursors, metabolic products influence the rate and the mechanism of the reactions and intracellular regulation.
c) Microorganisms can metabolize unconventional or even contaminated raw materials (cellulose, molasses, mineral oil, starch, wastewater, exhaust air, biogenic waste), a process which is frequently carried out in highly viscous, non-Newtonian media.
d) In contrast to isolated enzymes or chemical catalysts, microorganisms adapt the structure and activity of their enzymes to the process conditions, whereby selectivity and productivity can change. Mutations of the microorganisms can occur under sub-optimal biological conditions.
e) Microorganisms are frequently sensitive to strong shear stress and thermal and chemical influences.
f) Reactions generally occur in gas-liquid-solid systems, the liquid phase usually being aqueous.
g) The microbial mass can increase as the biochemical conversion progresses. Effects such as growth on the walls, flocculation, or autolysis of microorganisms can occur during the reaction.
h) Continuous bioreactors often exhibit complicated dynamic behavior.
Requirements of bioreactors
Due to the above-mentioned demands made by biological systems on their environment, there is no universal bioreactor. However, the general requirements of the bioreactor are as follows:
1) The vessel should be robust and strong enough to withstand the various treatments required such as exposure to high heat, pressure, and strong chemicals and washings and cleanings.
2) The vessel should be able to be sterilized and maintain stringent aseptic conditions over long periods of the actual fermentation process.
3) The vessel should be equipped with stirrers or mixers to ensure mass transfer processes occur efficiently.
4) It should have sensors to monitor and control the fermentation process.
5) It should be provided with an inoculation point for aseptic transfer in the inoculum.
6) Sampling valve for withdrawing a sample for different tests.
7) Baffles should be provided in case of stirred fermentor to prevent vertex formation.
8) It should be provided with a facility for intermittent addition of an antifoam agent.
9) In case of aerobic submerged fermentation, the tank should be equipped with an aerating device.
10) Provision for controlling temperature and pH of fermentation medium.
11) Manhole should be provided at the top for access inside the fermentor for different purposes. Fermentor design
Following basic points should be considered while designing a fermentor.
- Productivity and yield
- Fermentor operability and reliability
- Product purification
- Water management
- Energy requirements
- Waste treatment
Designing criteria
Design features so that process control will be possible over reasonable ranges of process variables.
- The operation should be reliable
- The operation should be contamination free.
- The traditional design is open cylindrical or rectangular vessels made from wood or stone.
- Most fermentation is now performed in a closed system to avoid contamination.
- Since the fermentor has to withstand repeated sterilization and cleaning, it should be constructed from non-toxic, corrosion-resistant materials.
- Small fermentation vessels of a few liters capacity are constructed from glass and/or stainless steel.
- Pilot-scale and many production vessels are normally made of stainless steel with polished internal surfaces.
- A very large fermentor is often constructed from mild steel lined with glass or plastic, to reduce the cost.
- If the aseptic operation is required, all associated pipelines transporting air, inoculum, and nutrients for the fermentation need to be sterilizable, usually by steam.
- Most vessel cleaning operations are now automated using spray jets, which are located within the vessels. They efficiently disperse cleaning fluids and this cleaning mechanism is referred to as cleaning-in-place CIP.
- Associated pipework must also be designed to reduce the risk of microbial contamination.
- There should be no horizontal pipes or unnecessary joints and dead stagnant spaces where material can accumulate; otherwise, this may lead to ineffective sterilization. Overlapping joints are unacceptable and flanged connections should be avoided as vibration and thermal expansion can result in the loosening of the joints to allow ingress of microbial contaminants
- Butt-welded joints with polished inner surfaces are preferred.
- Normally, fermentors up to 1000 L capacity have an external jacket, and larger vessels have internal coils. Both provide a mechanism for vessel sterilization and temperature control during fermentation.
- Other features that must be incorporated are pressure gauges and safety pressure valves, which are required during sterilization and operation. The safety valves prevent excess pressurization, thus reducing potential safety risks. They are usually in the form of a metal foil disc held in a holder set into the wall of the fermentor. These discs burst at a specified pressure and present a much lower contamination risk than spring-loaded valves.
- For the transfer of media, pumps are used. However, pumps should be avoided if the aseptic operation is required, as they can be a major source of contamination. Centrifugal pumps may be used, but their seals are potential routes for contamination. These pumps generate high shear forces and are not suitable for pumping suspensions of shear-sensitive cells.
- Other pumps used include magnetically coupled jet and peristaltic pumps.
- Alternate methods of liquid transfer are gravity feeding or vessel pressurization.
- In fermentations operating at high temperatures or containing volatile compounds, a sterilizable condenser may be required to prevent evaporation loss. For safety reasons, it is particularly important to contain any aerosols generated within the fermentor by filter Sterilizing the exhaust gases.
- Also, fermentors are often operated under positive pressure to prevent the entry of contaminants.
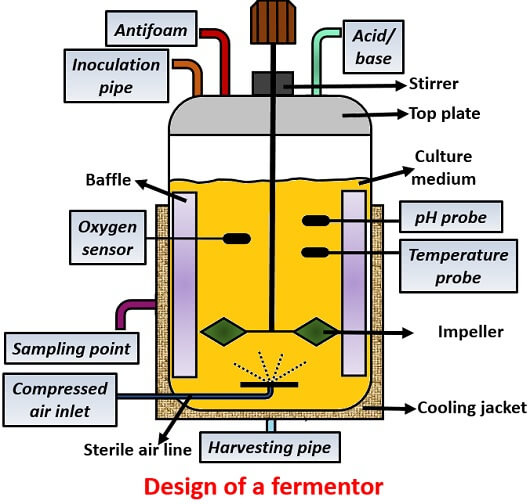
1) Material of construction
a) Laboratory-scale bioreactor
In fermentation with strict aseptic requirements, it is important to select materials that can withstand repeated sterilization cycles. On a small scale, it is possible to use glass and/or stainless steel. Glass is useful because it gives smooth surfaces, is non-toxic, corrosion-proof and it is usually easy to examine the interior of the vessel. The glass should be 100% borosilicate, e.g. Pyrex® and Kimax®.
b) Pilot-scale and large scale bioreactors
When all bioreactors are sterilized in situ, any materials used will have to assess their ability to withstand pressure sterilization and corrosion and their potential toxicity and cost. Pilot-scale and large-scale vessels are normally constructed of stainless steel or at least have a stainless steel cladding to limit corrosion.
The corrosion resistance of stainless steel is thought to depend on the existence of a thin hydrous oxide film on the surface of the metal. The composition of this film varies with different steel alloys and different manufacturing process treatments. The film is stabilized by chromium and is considered to be continuous, non-porous, insoluble, and self-healing. If damaged, the film will repair itself when exposed to air or an oxidizing agent.
2) Vessel shape
Stainless steel top plates
The top plates are of an elliptical or spherical dish shape. The top plates can be either removable or welded. A removable top plate provides the best accessibility but adds to cost and complexity. Bottom plates
Tank bottom plates are also customized for specific applications. Almost most of the large vessels have a dish bottom, while the smaller vessels are often conical in shape or may have a smaller, sump-type chamber located at the base of the main tank. These alternate bottom shapes aid in fluid management when the volume in the tank is low. One report states that a dish bottom requires less power than a flat one.
3) Height-to-diameter ratio (Aspect ratio)
The height-to-diameter ratio is also a critical factor in vessel design. Although asymmetrical vessel maximizes the volume per material used and result in a height-to-diameter ratio of one, most vessels are designed with a higher ratio. The range of 2-3:1 is more appropriate and in some situations, where stratification of the tank content is not an issue or a mixer is used, will allow a still higher ratio to be used in the design.
The vessels for microbiological work should have an aspect ratio of 2.5-3:1, while vessels for animal cell culture tend to have an aspect ratio closer to 1. In stirred tank bioreactor (STR), height to diameter aspect ratio is 3:1 or 4:1
4) Agitation
The agitator (impeller)
The agitator is required to achieve several mixing objectives such as – Bulk fluid and gas-phase mixing, air dispersion, oxygen transfer, and heat transfer,
Suspension of solid particles and maintaining a uniform environment throughout the vessel contents. Enhancement of mass transfer between dispersed phases. Bulk mixing and micromixing both are influenced strongly by impeller type, broth rheology. and tank geometry and internals. Impellers used bioreactors are Rushton disc turbines, vaned discs, open turbines of variable pitch, and propellers.
Baffles
To augment mixing and gas dispersion, baffles are employed. They are normally incorporated into agitated vessels of all sizes to prevent vortex and to improve aeration efficiency. Baffles are metal strips roughly one-tenth of vessel diameter and attached radially to the wall of the bioreactor. The agitation effect is only slightly increased with an increase in the width of baffles but drops sharply with narrower baffles. Generally, four to eight baffles are incorporated.
Baffles should be installed in such a way that a gap exists between them and the vessel wall so that there is scouring acting around and behind the baffles thus minimizing microbial growth on the baffles and fermentor walls. Extra cooling coils may be attached to baffles to improve the cooling capacity of the fermentor without affecting the geometry. With animal cells, culture baffles cause shear damage, instead of baffles bottom drive axial impellers slightly off the sight of the center is used.
The aeration system (sparger)
Gas under pressure is supplied to the sparger (usually either a ring with holes or a tube with a single orifice). It is defined as a device for introducing air into the liquid fermentor. Three basic types of sparger have been used and they are the porous sparger, the orifice sparger (a perforated pipe), and the nozzle sparger (an open or partially closed pipe). A combined sparger-agitator may be used in a laboratory fermenter.
The followings are adequate for good performance:
a) The sparge holes in the ring should be in line with the inner edges of the impeller blades.
b) The sparger holes should face downward to minimize medium retention in the sparger.
c) Hole diameter should be chosen such that each hole is a critical orifice at maximum glass flow.
d) The sparger inlet pipe should be placed to allow free draining back into the vessel.
Foam control
The problem often encountered in fermentation is foaming. It is very important to control foaming. When foaming becomes excessive, there is a danger that filters become wet resulting in contamination, increasing pressure drop, and decreasing gas flow. Foam can be controlled with a mechanical foam breaker or the addition of surface-active chemical agents, called anti-foaming agents. Foam-breaking chemicals usually lower KLa values, reducing the reactor’s capacity to supply oxygen or other gases, and in some cases, they inhibit cell growth.
The mechanical foam breaker available is “turbosep”, in which foam is directed over stationary turbine blades in a separator and the liquid is returned to the fermentor. Foam is also controlled by the addition of oils.
Control of foams by oil additions is of large economic importance to the fermentation industry. Excessive foaming causes loss of material and contamination, while excessive oil additions may decrease the product formation. Antifoam oils may be synthetic, such as silicones or polyglycols, or natural, such as lard oil or soybean oil. Either will substantially change the physical structure of foam, principally by reducing surface elasticity.
Industrial antifoam systems usually operate automatically from level-sensing devices.
Methods for metering of oil under aseptic conditions are:
i) timed delivery through a solenoid, two solenoids with an expansion chamber between,
ii) a motor-driven hypodermic syringe. and certain industrial pumps.
Temperature control
Normally in the design and construction of a fermentor, there must be adequate provision for temperature control which will affect the design of the vessel body. Heat will be produced by microbial activity and mechanical agitation and if generated by these two processes is not ideal for the particular manufacturing process then heat may have to be added to or removed from, the system. On a laboratory scale, little heat is normally generated and extra heat has to be provided by placing fermentor in a thermostatically controlled bath, or by use of internal heating coils or by a heating jacket through which water is circulated or a silicone heating jacket.
pH control
Certain microorganisms grow in particular pH only. In fermentation, it is very essential to control pH to grow the desired microorganisms for product formation. pH control sensors are used in fermentors for periodically checking pH.
Valves and steam traps
Valves attached to fermentors are used to control the flow of liquids and gases in a variety of ways. A wide range of valves are available, but not all of them are suitable for use in fermentor construction. These are also having a significant role in fermentor productivity.
The different valves available are gate valves, globe valves, piston valves, needle valves, plug valves, ball valves, butterfly valves, pinch valves, diaphragm valves, check valves, pressure control valves, safety valves, and steam traps.
Depending upon fermentation type and requirements these valves are chosen in designing bioreactors with good productivity.
Sampling port
On the one hand, sampling may seem to be a simple procedure – just open the manual valve in the inlet of the bioreactor vessel, supply as much fermentation broth as required for the sample, and close the tap. In this sampling, we can quite easily guarantee that infection will not be avoided. The sampling construction should be such so that measures for preventing non-sterility before and after the sampling are avoided. In the sites of the infection origin, sterilization should be performed promptly with alcohol or steam. The essence of sampling is based on the following
Disposable bioreactor
Recently available Disposable Bioreactors have good productivity compared to classical multiple-use bioreactors. Having following advantages.
- Pre-sterilized bag, no cleaning or sterilization is needed.
- Powerful and flexible Deltav based bioNet control system.
- All bag contact parts are single-use. class 4 tested and ready to use.
- Simplifies validation process.
- 50 through 1000 L working volumes.
- Scalable technology to support increasing volume demand.
- Minimizes investments and maximizes returns.
- A Single-Use Alternative to Conventional Stirred Tank Bioreactors
- Animal Derived Component Free (ADCF) Film
The Thermo Scientific HyClone S.U.B. (Single-Use Bioreactor) with BioNet®control provides all the advantages of single-use bioprocessing with the power and flexibility of DeltaV™ control.
The BioNet S.U.B. System is a turnkey product to replace the long lead time stainless Steel bioreactor vessels used previously. The combination of BioNet® and HyClone products results in a flexible, rapid, and powerful option to quickly update or increase your bioreactor capacity. The System consists of a reusable stainless steel outer support container and HyClone S.U.B. Bioprocess Container (BPC®) which integrate with the BioNet/DeltaV
You May Read: