Gram Staining
Gram staining is important and widely used technique. It is used to differentiate two large groups of bacteria based on their different cell wall components. The gram staining technique was developed by Dr. Christian Gram in 1884. Gram stain distinguishes between Gram-positive and Gram-negative groups by coloring these cells violet or pink. Gram-positive bacteria stain violet due to the thick peptidoglycan layer in the cell wall. Whereas, Gram-negative bacteria stain pink due to the thin peptidoglycan layer. This technique also allows the determination of cell morphology, size, and cell arrangement.
Principle and Mechanism of Gram Staining:
- In aqueous solutions, crystal violet dissociates into CV+ and CI– ions that penetrate through the wall and membrane of both Gram-positive and Gram-negative cells. The CV+ interacts with negatively charged components of bacterial cells, staining the cells purple.
- When added, iodine (I–) interacts with CV+ to form large crystal violet-iodine (CV-I) complexes within the cytoplasm and outer layers of the cell.
- The decolorizing agent (ethanol or an ethanol and acetone solution) interacts with the lipids of the membranes of both Gram-positive and Gram-negative bacteria. Since Gram-negative organism have a thin peptidoglycan layer (1-2 layers) and have an additional lipopolysaccharide layer that gets dissolved due to the addition of alcohol, so Gram-negative organism fails to retain the complex and gets decolorized as the complex is washed away.
- In contrast, a Gram-positive cell becomes dehydrated from an ethanol treatment. This closes the pores in the cell wall and prevents the stain from exiting the cell. The large CV-I complexes become trapped within the Gram-positive cell also due to the thick and multi-layered (40 layers) nature of its peptidoglycan.
- After decolorization, the Gram-positive cell remains purple and the Gram-negative cell loses its purple color. Counterstain, which is usually positively charged Safranine or Basic fuchsin, is applied last to give decolorized Gram-negative bacteria a pink or red color.
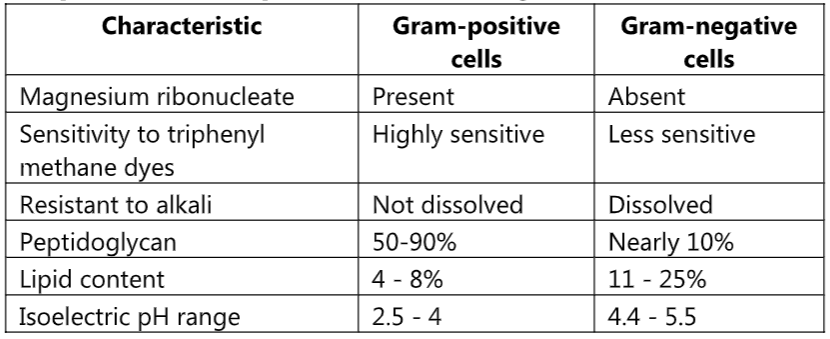
Comparison of Gram-positive and Gram-negative Cell Wall
Theories of Gram Staining
(A) Lipid Content Theory:
In the case of Gram-negative bacteria cell wall contains a higher percentage of lipids which is 11% to 25%. When we apply alcohol (ethanol) as a decolorizer, these lipids get dissolved in it. The permeability of the cell wall increases and the CV-I complex gets easily extracted. Therefore, the cell becomes colorless and accepts secondary stains i.e. safranine. Gram-positive bacteria contain less amount lipid (4% to 8%) in their cell wall. When ethanol is applied cells are dehydrated and shrinkage occurs. Pore size decreases and the CV-I complex is retained inside the cell so they appear violet in color due to crystal violet.
(B) Peptidoglycan Theory:
Gram-positive bacteria have a thick mesh-like cell wall that is made up of peptidoglycan (50-90% of the cell wall), which stains purple. Peptidoglycan is mainly a polysaccharide composed of two subunits called N-acetyl glucosamine and N-acetyl muramic acid. As adjacent layers of peptidoglycan are formed, they are cross-linked by short chains of peptides using a transpeptidase enzyme, resulting in the shape and rigidity of the cell wall. The thick peptidoglycan layer of Gram-positive organisms allows these organisms to retain the crystal violet-iodine complex and stains the cells purple.
Gram-negative bacteria have a thinner layer of peptidoglycan (10% of the cell wall) and lose the crystal violet-iodine complex during decolorization with the alcohol rinse, but retain the secondary or counterstain Safranine, thus appearing reddish or pink.
(C) Magnesium Ribonucleate Theory:
Magnesium ribonuclease is present only in Gram-positive bacteria with which CV-I complex forms a covalent bond. Therefore, the CV-I complex is retained and cells appear violet in color.
(D) Stearn and Stearn Theory:
According to them lipoidal content of Gram-positive bacteria contains a large amount of unsaturated fatty acids. These fatty acids are having more affinity for oxidizing agents (Gram’s iodine). The oxidizing product of lipids is more acidic. Therefore, acidic conditions developed which results in increased affinity for basic stain. In Gram-negative bacteria, less amount of unsaturated fatty acids so an acidic condition is not developed therefore they have less affinity for primary stain.
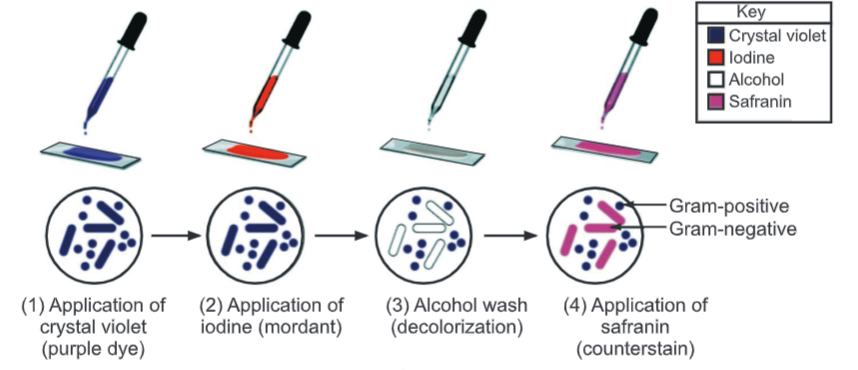
Procedure:
- Prepare a smear with a loopful of culture suspension/sample on a clean, dry, and grease-free slide. Air-dry the smear.
- Heat fix the smear by passing lightly through the flame of the Bunsen burner.
- Place the slide on the staining rods.
- Cover the smear with a crystal violet stain and leave for 1 minute.
- Wash carefully under running tap water.
- Flood the smear with Gram’s iodine (used as mordant) solution and leave for 1 minute to form a crystal violet-iodine complex.
- Drain off the iodine. Wash the slide again in a gentle stream of tap water.
- Flood the slide with the decolorizing agent then waits for 20-30 seconds. This can also be done by adding a drop by drop to the slide until the decolorizing agent running from the slides runs clear.
- Gently wash the slide under running tap water and drain completely.
- Counterstain with Safranine and wait for about 30 seconds to 1 minute.
- Wash slides in a gentle and indirect stream of tap water until no color appears in the effluent and then blot dry with absorbent paper.
- Observe under the microscope.
Video Procedure
Factors Affecting Gram Staining:
1. Age of culture: Old cultures of Gram-positive bacteria lose the ability to retain primary stain. Therefore, even though they are Gram-positive they may appear pink. So, the culture should be 18 – 24 hours old.
2. Excessive heat fixation: Too much heating results in loss of Gram positivity.
3. Overcrowding: If the number of bacteria present in the smear is very much high, every cell will not come in contact with a decolorizer which may result in a wrong interpretation. The smear should be thin.
4. Old staining reagents: Old staining reagents may give wrong results. Because they deposit on the surface of bacteria, so the staining reagents should be freshly prepared.
5. Air drying: For good results, first air drying and then heat fixation is done. If a wet smear comes directly in contact with heat, there will be charring of bacteria and total distortion of shape and structure take place.
Examples of Gram-Positive Organisms: Bacillus, Nocardia, Clostridium, Propionibacterium, Actinomyces, Enterococcus, Corynebacterium, Listeria, Lactobacillus, Gardnerella, Mycoplasma, Staphylococcus, Streptomyces, Streptococcus, etc.
Examples of Gram-Negative Organisms: Escherichia, Helicobacter, Hemophilus, Neisseria, Klebsiella, Enterobacter, Chlamydia, Vibrio, Pseudomonas, Salmonella, Shigella.
