Contents:
Screening and its Techniques
Screening may be defined as the use of selection procedures for isolation of high yielding species from natural sources, such as soil, water, plants and animals containing a heterogeneous large microbial population.
Screening techniques are mainly classified as primary screening and secondary screening.
Primary screening:
Primary screening consists of some preliminary tests required to isolate microbial species exhibiting bioactive properties. These tests are performed for the isolation of microorganisms capable of producing antibiotics, organic acids, enzymes and vitamins. Some of the techniques used for primary screening are described as follows:
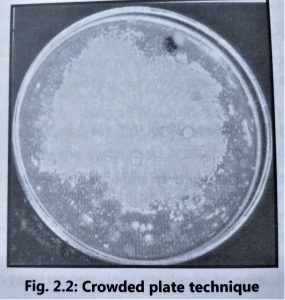
1. Crowded plate technique:
- The crowded plate technique is the simplest screening technique used for the isolation of antibiotic-producing microorganisms.
- Soil or other sources of microorganisms are diluted in sterile saline solution and then spread on nutrient agar plates by using the spread plate technique.
- The agar plates having 300 or more colonies per plate are selected because they are helpful in locating the colonies producing antibiotic activity.
- Microorganisms producing antibiotic activity are indicated by a zone of inhibition around the colony (Fig. 2.2).
- Antibiotic producing colonies are subcultured by using a similar medium.
- All active colonies are purified by the streak plate technique, before making stock cultures. It is necessary to carry on further testing to confirm antibiotic activity associated with a micro-organism because the zone of inhibition can be occurred by other causes such as acid production by the microorganism which can alter the pH of the medium thus inhibiting the growth of other organisms nearby.
- The crowded plate technique provides information regarding the inhibitory activity of a colony against unknown microorganisms.
- It is necessary to confirm activity against specific microorganisms. Therefore, the crowded plate technique has been modified by introducing a ‘test organism’.
- Dilutions of soil or other sources are spread on the surface of agar plates so that isolated colonies are developed (approx.50 to 200/plate).
- After the development of well-isolated growth, test organism suspension is spread on the same agar plate.
- The plates are again incubated for the growth of test microorganisms. The formation of inhibitory zones around certain colonies indicates their antimicrobial activity.
- Antibiotic producing colonies are again isolated and purified before preservation and further testing.
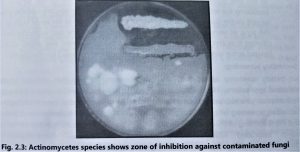
2. Accidental contamination method:
- In these techniques, plates are further incubated for the study of growth characteristics of newly isolated microbes.
- These plates are accidentally contaminated by environmental microorganisms. If these contaminated plates are further incubated for 2 to 5 days then the isolated microbes(those having antimicrobial activity) show a zone of inhibition against the colonies(Fig. 2.3).
 3. Enrichment culture technique:
3. Enrichment culture technique:
- In this method, medium composition or incubation conditions have adjusted that favour the growth of desired microorganisms.
- Undesired microorganisms are not developed because they do not find suitable growth conditions.
- It is necessary to screen microbial sources in order to find microorganisms capable of utilizing specific carbon and nitrogen sources.
- Soil samples are usually heated at 80 to 100°C for 10 to 20 minutes for isolation of actinomycetes, fungi and other spore carrying microorganisms.
- These spore carrying species are mainly isolated for the production of antibiotics, enzymes and other bioactive metabolites. Preheat treatment kills vegetative cells but spores are unaffected.
- Heat-treated samples are diluted and spread onto the surface of nutrient agar containing casein at pH 10 to 12.
- The colonies surrounded by a clear zone are isolated, purified and then subcultured. This clear zone indicates that the particular species excrete alkaline protease which degrades casein.
4. Indicator dye method:
- The pH indicating dyes mainly employed for detecting microorganisms are capable of producing organic acids or amines e.g. neutral red, bromothymol blue etc.
- These pH indicating dyes are added into the poorly buffered nutrient agar media which undergoes colour changes according to its pH.
- The change in the colour of a dye in the vicinity of the colony suggests the capability of cells to produce organic acids or amines.
- The production of organic acids m detected by the addition of calcium carbonate in the medium.
- Acid production is indicated by a cleared zone of dissolved calcium carbonate around the colony
5. Auxanography:
- This technique is largely employed for detecting microorganisms capable of synthesizing extracellular vitamins, amino acids or other growth-stimulating metabolites.
- Dilutions of soil are applied to agar plates which produce well-isolated colonies. Another agar plate contains the test organism.
- The agar layer of the first plate is aseptically transferred on the surface of the second plate containing the test organism.
- Vitamins and amino acids producing colonies present (in soil) on the surface of the first layer of agar can diffuse in the lower layer of agar.
- The zone of stimulated growth (zone of the exhibition) of the test organism near the colonies indicates that these colonies may produce vitamins and amino acids.
- The isolated active colonies are purified, sub-cultured and retested for metabolites.
Secondary screening:
- Primary screening allows the isolation of microorganisms that possess important industrial applications.
- This technique does not provide much information related to the fermentation process, production or yield potential of the isolated new microorganisms.
- Secondary screening helps in the detection of useful microorganisms in fermentation processes and the discarding of those lacking industrial potential.
- It is necessary to know the following points associated with secondary screening for isolation, standardisation and characterisation of new bioactive producing microorganisms.
- Secondary screening is very useful for the separation of new microbial cultures that have real industrial applications from isolates obtained in primary screening.
(i) It provides information about whether the microorganisms are producing new chemical compounds or not. This may be confirmed by comparison with known compounds using thin layer, paper or other chromatographic techniques.
(ii) It helps in providing information regarding the product yield of newly isolated microorganisms. This information is very useful in selecting specific cultures for the final fermentation process.
(iii) Secondary screening reveals that the pH, temperature, aeration or other critical requirements associated with bioactive microorganisms for the growth of particular microbes and for the formation of new chemical products. It may give an idea whether specific medium constituents are missing or some constituents may produce toxic effects on the growth of microorganisms.
(iv) The screening techniques detect the genetic instability of isolated new microbial species, Many microbial species may lose their capability for maximum production of active components by the process of mutation or recombination.
(v) Physical, chemical and biological properties of a product are determined by secondary screening. It gives an idea about the chemical stability of products and solubility of products in various organic solvents.
(vi) It reveals whether a product produced from fermentation occurs in the culture broth in more than one chemical form. Two or more different bioactive compounds are also produced by a single fermentation.
(vii) It provides information about new products. The product may have a simple, complex or macromolecular structure.
(viii) Many fermentation products (mainly antibiotics) are tested for toxicity to animal, plant or human beings. These techniques give information about the toxicity of products that are mainly used for therapeutic purposes.
(ix) It reveals whether microorganisms are able to chemically alter or destroy their own fermentation products. The microbial cultures may produce adaptive enzymes that destroy the usefulness of the product. They may produce a ‘racemase’ enzyme that changes the L-configuration of an amino acid product to a mixture of the D and L-isomers.
- Thus, secondary screening gives answers to many questions that arise during the isolation of bioactive molecules from natural sources.
- Secondary screening is mainly performed on agar plates, in flasks or small scale fermenters containing liquid media or, by a combination of these approaches.
- Streptomyces species are mainly considered for an understanding of the sequence of events during a screening programme.
- Streptomyces is a well-known genus for the production of antibiotics, enzymes and other active molecules mainly isolated from soil, water and other natural sources.
- All primarily isolated species are screened for antibiotic production on agar media. Species exhibiting antimicrobial activity are subjected to an initial secondary screening.
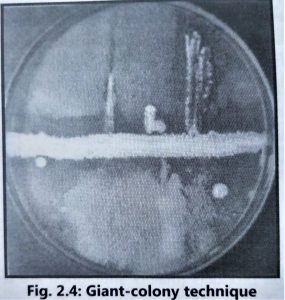
- A simple ‘giant colony technique’ is used to determine the initial ‘inhibition spectrum’ for the antibiotics produced by each Streptomyces species (Fig. 2.4).
- The Streptomyces isolates are inoculated on starch casein agar or any other suitable agar media as a straight line and incubated at 28 to 30°C for 5 to 7 days.
- After proper growth, different test microorganisms such as Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa, Aspergillus niger, Aspergillus fumigatus, Candida albicans, Penicillium species and Cryptococcus species are cross-streaked at right angles to the border of the actinomycetes. Plates are further incubated at 35-37″C for 24 to 48 hours if the test organism is bacteria and at 25-28°C for 3 to 4 days for fungal test organisms.
- After incubation, growth inhibitory zones for each test microorganism are measured in millimetres. Streptomyces species that have produced antibiotics with interesting microbial inhibition spectra are retained for further testing.
- Antimicrobial screening is carried out by using liquid media in flasks. These broth studies give more information than what can be obtained on agar media.
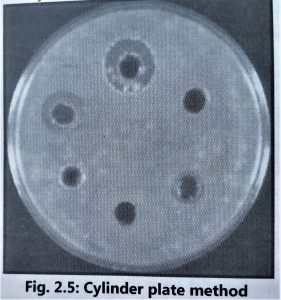
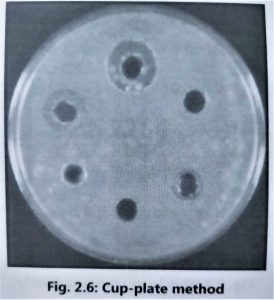
- It is advisable to use the cylinder plate method (Fig. 2.5) and the cup-plate method (Fig. 2.6) to determine the exact amount of antibiotic present in isolated culture fluids.
- Actinomycetes isolates are studied by using different liquid media in Erlenmeyer flasks provided with baffles.
- The media are sterilised and inoculated with one of the Streptomyces species to be tested.
- The flasks are shaken at a constant temperature, usually near room temperature by keeping them on a mechanical shaker.
- Streptomycetes growth and antibiotic production are better in aerated flasks than the stationary ones.
- In some cases (e.g. polyene antibiotics), the antibiotic activity is present not in the culture fluid but within the mycelium of Streptomyces species (intracellular).
- Thus, such cases require special extraction procedures for recovering antibiotics from the mycelium. In the present discussion, we have considered only those species that produce antibiotics in the culture fluids (extracellular). Samples are withdrawn at regular intervals under aseptic conditions for analysis.
- The samples are checked for the presence of contamination by microscopic observation and by the addition of inoculum into the medium.
- The pH value of the sample is also determined for the proper growth of the Streptomyces species during antibiotic production.
- The culture samples are adjusted to a pH value near neutrality and assayed for antibiotic production against a sensitive test microorganism.
- These techniques give information about the best medium and growth phase for antibiotic production.
- Samples are analysed by paper or thin-layer chromatography to determine the antibiotic is similar to previously known antibiotics or whether there is more than one antibiotic present in the culture broth (Fig. 2.7).
- It is also important to compare the microbial inhibition spectrum of the liquid and agar grown Streptomyces species and expand the spectrum by using viruses and carcinoma agents.
- The solubility of the antibiotic in various organic solvents is determined by solvent extraction of culture filtrate.
- The antibiotic is purified by column chromatography and the structure is determined by IR, NMR, mass and elemental analysis. Purified antibiotic material is tested for toxicity in mice or other laboratory animals. It is also tested for adverse effects on plant, animal or human beings.
- Some isolated species of Streptomyces may not produce valuable new antibiotics and such species are discarded.
- Further studies are required that yield additional information about fermentation media, microbial species and the antibiotic. Similar techniques are employed for the isolation of new strains from natural sources for the production of different bioactive molecules.
- For the enhancement of antibiotic yields, different strain improvement techniques are used.