Contents:
Aim: To study metachromatic granule staining
Introduction:
Albert’s staining is used to demonstrate metachromatic granules. Albert’s staining describes a special structure of metachromatic granules in Corynebacterium diphtheria. Corynebacterium causes the disease diphtheria. The name Corynebacterium is derived from the Greek word Coryne, it means club shape of bacteria. Metachromatic granules are storage granules that show the property of metachromasia. It also has the property of changing colour i.e. when stained with blue stain they appear red in colour. The granules appear in a colour other than the colour used for staining. The granules are made up of poly-meta-phosphates and are also referred to as volutin granules, Babe-Ernst granules or polar bodies. Bacillus strains green and granules stain bluish black when Albert’s stain is applied.
Albert’s stain is basically made up of two stains, Toluidine blue O and Malachite green. Both are basic stains with a high affinity for acidic tissue components like cytoplasm. Albert’s A solution consists of Toluidine blue, malachite green, glacial acetic acid and ethyl alcohol while Albert’s B solution consists of iodine and potassium iodide.
Principle:
Albert’s stain is basically made up of two stains, Toluidine blue O and Malachite green. Both are basic stains with a high affinity for acidic tissue components like cytoplasm. Albert’s A solution consists of Toluidine blue, malachite green, glacial acetic acid and ethyl alcohol while Albert’s B solution consists of iodine and potassium iodide. The pH of Albert’s stain is adjusted acidic which becomes basic for metachromatic granules which are highly acidic. When Albert’s stain is applied on the smear, Toluidine blue O stains metachromatic granules. Due to the application of Albert’s iodine metachromatic property is not visible and granules appear blue in colour. Cell shows acidic property and malachite green stains the cytoplasm, which appear blue-green in colour.
Requirements:
- Chemicals: Albert’s A solution, Albert’s B solution, Immersion oil.
- Apparatus: Glass slide, Staining rack, Filter paper, Microscope
Preparation of Albert’s Stain
Albert’s solution A:
Composition:
- Toluidine blue -0.12gm
- Malachite green- 0.20gm
- Glacial acetic acid- Iml
- Alcohol (95%) – 2ml
Procedure:
i. Dissolve the dyes in alcohol and add fo the Distilled water and acetic acid.
ii. Filter the stain and allow for one day.
iii. Add distilled water to make the final volume 100ml
b. Albert’s solution B:
Composition:
- Iodine -2gm
- Potassium iodide (KT) – 3gm
Procedure:
i. Dissolve KI in water
ii. Add iodine to the above solution and dissolve.
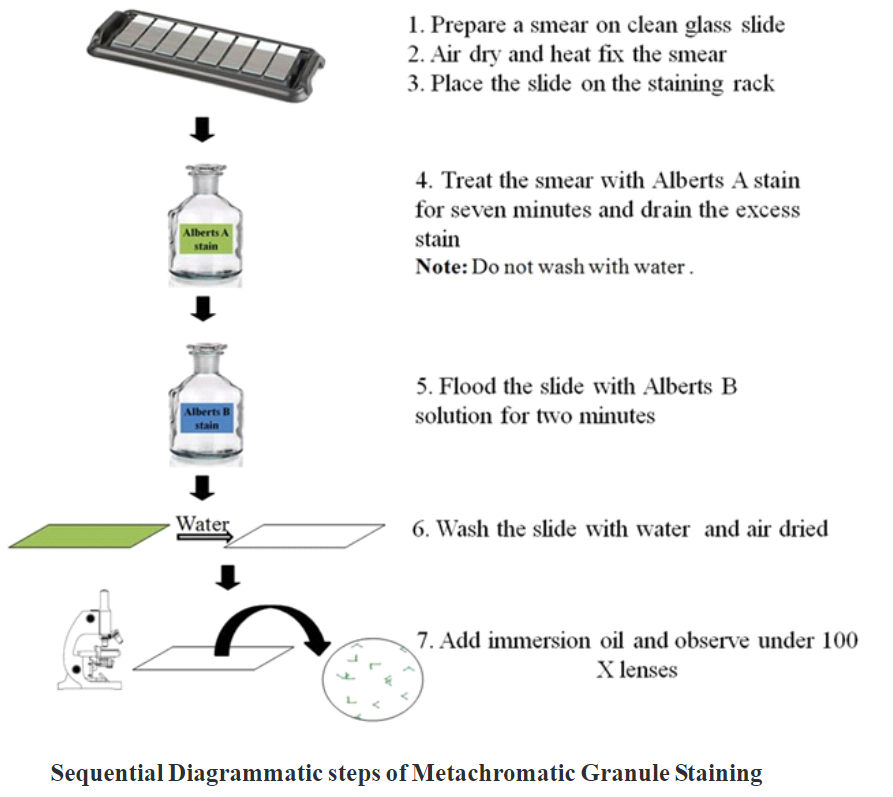
Procedure:
- Prepare a smear on a clean grease-free glass slide.
- Airdry and heat the slide to fix the smear.
- Place the slide on the staining rack.
- Treat the smear with Albert’s A stain for 7 minutes.
- Drain the excess stain and do not wash with water.
- Flood the slide with Albert’s B solution for 2 minutes.
- Wash the slide with water and air dry it.
- Dry the slide using blotting paper, place a drop of immersion oil on the smear and observe
under the oil immersion objective.
Observation:
Check whether Corynebacterium diphtheria is present in the sample. Observe the shape, size and colour of metachromatic granules at the poles.
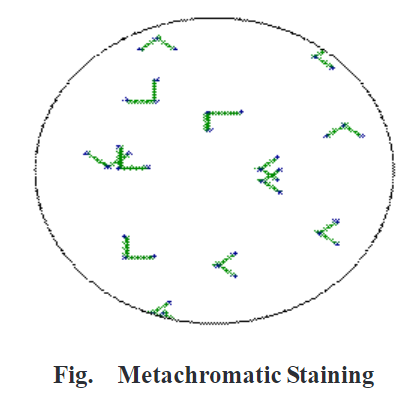
Result:
Corynebacterium diphtheria is present in the sample, green coloured rod-shaped bacteria is present in resembling English letter L, V and show bluish-black colour metachromatic granules at the poles. Corynebacterium diphtheria appears in club shape and swells up at the ends. The granules appear bluish-black in colour and cells appear green.
Application:
Metachromatic granule staining helps to distinguish Corynebacterium diphtheria from non pathogenic diphtheroid which lacks granules.
Key Points:
- The smear should be thin and even.
- Gentle water must be used for washing treatment. Do not apply forceful water so bacteria from the smear will not be washed away.
- The stain should be applied in proper proportion.
YOU MAY READ:
- Negative Staining: Principle, Procedure, Results and Application
- Flagella Staining: Principle, Procedure and Example
- Endospore Staining: Principle, Procedure, Results and Example
- Gram Staining: Principle, Theories, Procedure and Examples
